Genetic Variation In Taste Receptors and Eating Behavior
Course Authors
Hillary L. Shaw, M.D., Cedrick D. Dotson, Ph.D., Steven D. Munger, Ph.D., and Nanette Steinle, M.D.
Hillary L. Shaw is a postdoctoral trainee in the Department of Medicine, Caritas Carney Hospital, Dorchester, MA; Cedrick D. Dotson is a postdoctoral fellow in the Department of Anatomy and Neurobiology, Steven D. Munger is an Associate Professor of Anatomy and Neurobiology and Nanette Steinle is an Assistant Professor of Medicine, all at the University of Maryland School of Medicine, Baltimore, MD.
Within the past 12 months, Drs. Shaw, Dotson and Steinle report no commercial conflicts of interest. Dr. Munger Dr. Munger has been a consultant with McNeil Nutritionals and has done research for Ajinomoto Amino Acid Research Program.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Describe behaviors associated with genetic variation in taste receptors
Identify the receptor type most commonly associated with ingestive behavior
Identify individuals whose eating behavior is likely to be most influenced by genetic variation in taste receptors
Identify biologic processes involved in taste receptor modulation of eating behavior.
Taste perception influences food preference and intake. Sensory inputs from taste receptors provide information regarding the identity and flavor of food. (1)(2) Taste sensations can induce satiety and reinforce eating. (3)(4)(5)(6) Taste bud density, differences in salivary constituents, genetic variation in taste receptors, and exocrine and endocrine factors may all influence food intake and, therefore, influence risk for nutrition-related conditions including obesity, diabetes, cardiovascular disease, hypertension, hyperlipidemia and cancer.
There are five widely accepted taste perceptual qualities: sweet, bitter, sour, salty and umami (the savory taste of glutamate). Sweet-, umami- and bitter-tasting stimuli are detected by distinct members of the seven-transmembrane, G protein coupled receptor (GPCR) family (Figure 1).
There are five widely accepted taste perceptual qualities: sweet, bitter, sour, salty and umami.

Figure 1. Schematics of TAS1R (sweet and umami) and TAS2R (bitter) Taste Receptors.
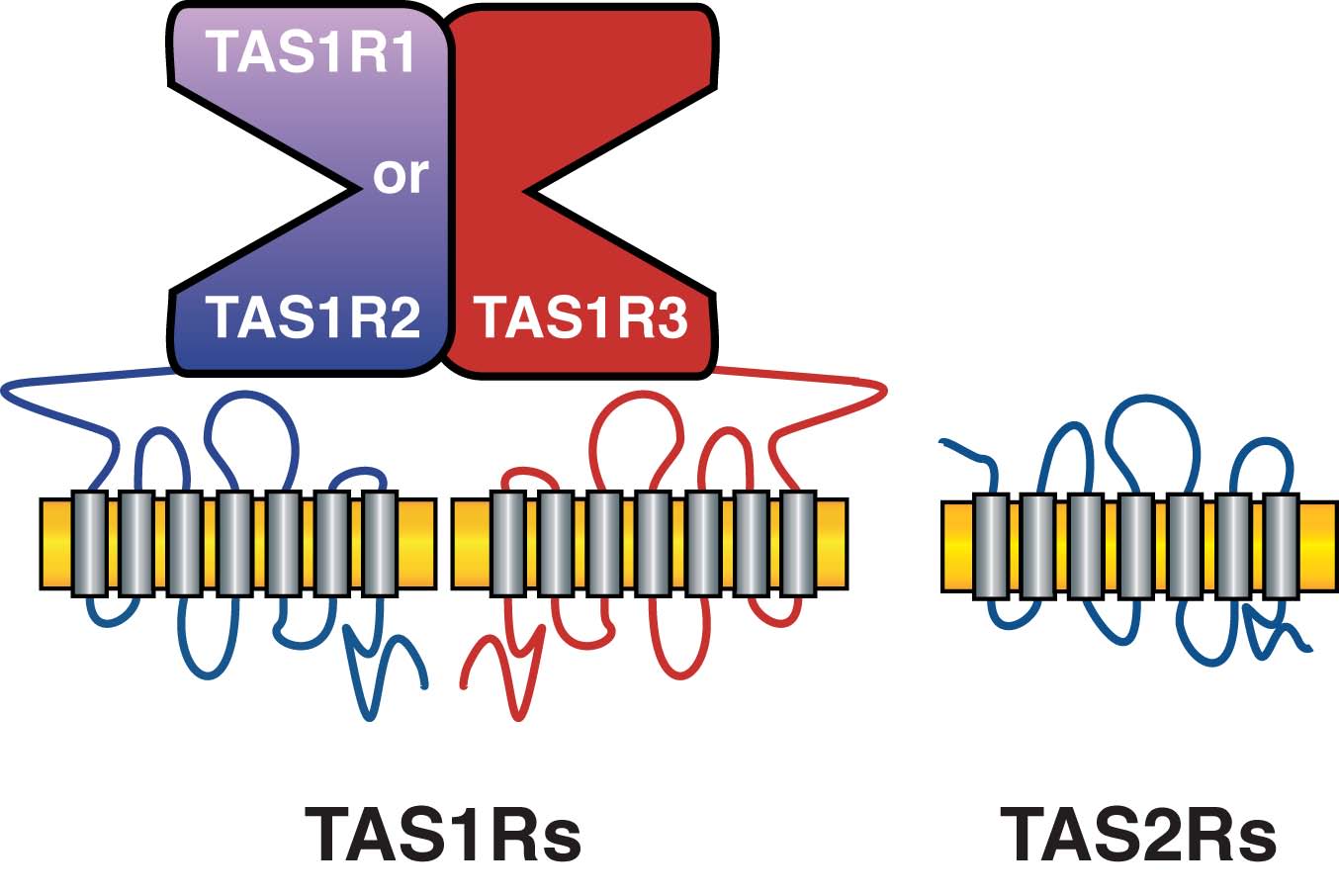
Courtesy of S. Munger
TAS1Rs function as heteromeric receptors: the umami receptor contains TAS1R1 and TAS1R3, while the sweet receptor contains TAS1R2 and TAS1R3. Most TAS1R ligands bind within the large extracellular N-terminal domain, while TAS2Rs likely bind bitter ligands within the transmembrane domains.

The receptors for sour- and salty-tasting compounds have not been identified, but may be ion channels. Other taste qualities have been suggested (e.g., fat, calcium), but it is unclear if these represent distinct tastes or whether they modulate other taste qualities. Sweet and umami taste can signal the presence of essential, energy rich nutrients and can promote food intake in humans. In contrast, bitter taste may warn of the presence of potential toxins in foods,(7) while sour taste could alert us to the potential of food spoilage.(8) Salt taste mediates the ingestion of NaCl and other essential minerals, thus playing a vital role in ion and water homeostasis;(8) however, intake of high concentrations of salts, which could lead to dehydration and renal damage, are aversive to most individuals with intact taste.
Taste receptors are expressed on the plasma membrane of taste cells, which are clustered in structures called taste buds. Taste buds, in turn, are associated with fungiform, foliate and circumvallate papilla on the anterior, lateral and posterior aspects of the tongue, repectively, as well as scattered in the oral epithelia of the soft palate. Taste receptors act as molecular sensors of the chemical environment.

Figure 2. Taste Buds in Circumvallate Papillae.
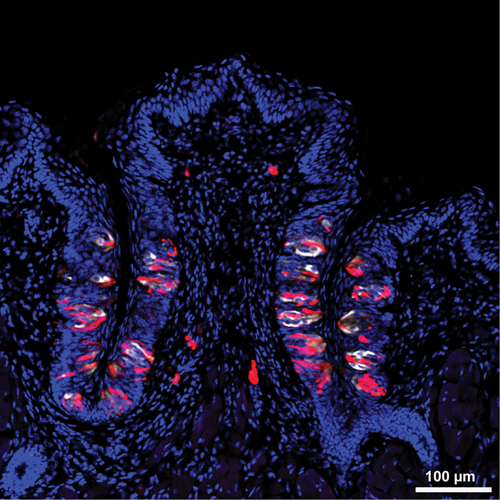
Several onion-shaped taste buds are found in the trenches around the circumvallate papillae of the mouse. Subsets of taste cells are identified by immunohistochemical staining for phospholipase C β2 (red) or for serotonin (white). Blue, DAPI-stained cell nuclei. Courtesy Elson and Munger.

Two gene families within the GPCR superfamily, TAS1R and TAS2R, encode sweet, umami and bitter taste receptors in humans and other mammals.
Stimulation of taste receptors activates an intracellular transduction cascade, changes in membrane potential and the release of neurotransmitter onto associated nerve fibers, which carry taste information to the central nervous system. The density of fungiform papillae is thought to be genetically determined.(9) Individuals with a high density of fungiform papillae experience more intense sensations from taste and oral stimuli, likely because they have more taste buds and thus more taste receptor cells. Indeed, taste perception, in general, is thought to be under strong evolutionary selection.
Two gene families within the GPCR superfamily, TAS1R and TAS2R, encode sweet, umami and bitter taste receptors in humans and other mammals. The TAS1R family consists of three genes, TAS1R1, TAS1R2 and TAS1R3. TAS1R2 and TAS1R3 proteins associate as a single complex to form the sweet taste receptor,(10)(11) while TAS1R1 and TAS1R3 combine to form the umami taste receptor.(10) Bitter taste is mediated by ~25 TAS2R receptors. TAS1Rs and TAS2Rs are expressed in distinct subsets of taste receptor cells of the gustatory epithelium. They are also found in specialized cells in the gastrointestinal (GI) tract,(12)(13)(14) including enterocytes(15)(17) and enteroendocrine cells.(15)(18) Thus, stimulation of TAS1Rs or TAS2Rs in the GI tracts can result in adaptive resposes independent of taste perception. For example, in enteroendocrine cells, taste receptor agonists elicit peptide hormone secretory response, as well as neuronal activation of vagal afferents.
The physiologic response at the cellular level to activation of a cell via TAS1Rs and TAS2Rs involves intracellular release of calcium and activation of calcium signaling pathways. These GPCRs act in part via G proteins, including α-gustducin. In the oral cavity stimulation of TAS1Rs and TAS2Rs results in neurotransmitter release and stimulation of brain centers and the detection of taste.(8) In the GI tract, TAS1R stimulation activates the expression of the glucose transporter GLUT2 in enterocytes(17) and results in GLP-1 release from enteroendocrine L cells(14)(17) as well as cholecystokinin (CCK) release from enteroendocrine K cells.(19) Stimulation of TAS2Rs in human enteroendocrine cells also results in GLP-1 secretion.(18)
Strong evidence to support variation of individual responses to sweet compounds is scant.
TAS1Rs and Sweet and Umami Taste
Strong evidence to support variation of individual responses to sweet compounds is scant. This may reflect modest inter-individual differences in sweet perception or somewhat weak test-repeat reliability for sweetness detection thresholds.(20) On the other hand, there is some support for variation in taste sensitivity to L-glutamate, an umami stimulus. A small number of non-tasters have been identified.(21) However, the distribution in taste sensitivity among humans appears to be multimodal, suggesting the involvement of multiple transduction mechanisms in umami taste.(21) Indeed, isoforms of some metabotropic glutamate receptors have been suggested as additional umami taste receptors.(22) One study identified 47 distinct single nucleotide polymorphisms (SNPs) in TAS1R genes, 29 of which cause amino acid substitutions.(23) However, TAS1Rs exhibit much less sequence diversity than do TAS2Rs (see below), which may account for the higher degree of stability in sweet and umami taste in humans.
TAS2Rs and Bitter Taste
The current human genome annotation suggests there are approximately 25 functional TAS2R genes clustered on chromosomes 12p13, 7q34 and 5p15.31. Most TAS2Rs are broadly tuned to multiple stimuli, consistent with a role in detecting thousands of bitter-tasting compounds.(7) One of these receptors, TAS2R38, has been extensively characterized in vitro, in vivo and in human populations. TAS2R38 is responsive to a variety of bitter-tasting stimuli containing the N-C=S (thiocyanate) moiety [e.g., phenylthiocarbamide (PTC) and propothiouracil (PROP), as well as several bitter compounds in brassica vegetables]. Two common TAS2R38 haplotypes, the "taster" haplotypes PAV and the "nontaster" haplotypes AVI, have been shown to influence perception of these compounds. The PAV and AVI haplotypes are both prevalent in human populations, but this prevalence varies between different ethnic groups: among individuals of Northern European decent, PAV and AVI TAS2R38 haplotypes represent 49% and 47% of all haplotypes, respectively, while they represent 70% and 30% of TAS2R38 haplotypes in individuals of East Asian decent.(24) Although PROP taster status is also studied with respect to bitter taste from a variety of compounds, TAS2R38 is not a specific marker for general bitter taste sensitivity.(25)(26)
Bitter taste perception affects food preference and intake.(28)(29)(30)(31)(32) A general bitter "taster" phenotype, where individuals perceive all bitter stimuli more intensely than non-tasters, is often confused with the TAS2R38-dependent PROP-taster phenotype, as this compound is often used in human taste testing to assess general bitter taste sensitivity. Rather, hypersensitivity to stimuli of all taste qualities (including bitterness), as well as to oral irritation and the general sensations of foods in the mouth, (for example, texture, hardness, softness, crispiness), is more likely a consequence of increased numbers of taste papillae (and associated taste cells); individuals with this phenotype are known as "supertasters." Thus, one should be cautious to extrapolate PROP or PTC taste sensitivity to differences in TAS2R38 haplotype.
Bitter taste perception affects food preference and intake.
PROP taster and nontaster phenotypes have been associated with preference for sucrose and sweet tasting foods and beverages, with tasters having reduced preference for sweets.(33)(34) Differences in bitter taste perception have also been associated with preferences for high-fat food,(31)avoidance of specific fruits and vegetables,(35)(36)(32) alcohol intake, and tobacco use.(37)(38) PROP tasters are more sensitive to bitterness from beer,(39) alcohol (40)(41)(37) and the astringency of red wine.(42) In the study reported by Wang et al., the common PROP taster haplotype was significantly associated with lower mean alcohol consumption compared with other haplotypes.(43) Genetic variation in another bitter receptor, TAS2R16, has also been linked to alcohol dependence, with the at risk allele being observed more frequently among African Americans.(44) The TAS2R38 taster haplotype PAV is associated with smoking quantity in African American smokers. It has been suggested that heightened oral sensitivity may confer protection against nicotine dependence.(45)
Eating behavior is a heritable trait.(46)(47)(48)(49)(50) A commonly used tool to measure eating behavior traits is the Three Factor Eating Questionnaire.(51)(52) Initially designed to measure eating behavior in dieting women, it measures three scales: Cognitive Restraint, Hunger and Disinhibition. Restraint is the cognitive avoidance of eating to control body weight. An example of a question from the eating questionnaire that addresses restraint is, "I do not eat some foods because they make me fat." Disinhibition is loss of restraint resulting in overeating. An example of a disinhibition question is, "When I am with someone who overeats I usually overeat too." Hunger measures the perceived need for food, e.g., "I get so hungry that my stomach often feels like a bottomless pit."(51) The heritability estimates for hunger from family and twin studies using the Three Factor Eating Questionnaire reach approximately 8-28%; estimates for disinhibition reach 18-45%, and for restraint 6-58%. Although there is considerable variation in the magnitude of genetic variance reported in these studies, the preponderance of evidence suggests significant genetic contribution exists pertaining to these traits. Disinhibition and hunger are generally positively correlated with obesity, while restraint is negatively correlated with body mass index (BMI) and body fat.(53)(54)(55)(56)
In studies designed to detect associations between taste sensitivity and obesity phenotypes, eating behavior traits (e.g., dietary restraint and disinhibition) significantly impacted the relationship between these phenotypes.(57)(58) Recently, Tepper and colleagues have shown that women who lack sensitivity to PROP have higher BMI and waist circumference compared to those who were phenotypic tasters, although TAS2R38 haplotype did not account for this association.(58) This relationship was specific to females with low dietary restraint.(58) However, a study performed in children revealed PROP tasters had significantly higher BMI z-scores as compared to nontasters.(59) Another study showed the same relationship among boys, but not girls.(60) Observations from our group suggest that TAS2R genetic variants are associated with disinhibition in women but not men (*our unpublished data).
How might genetic variation in taste receptors influence eating behavior? In addition to influencing taste perception, TAS1Rs and TAS2Rs can modulate nutrient uptake and/or gut hormone release. Functional variants in these receptors may impact nutrient assimilation and prandial hormone response. For example, stimulation of TAS1Rs and/or TAS2Rs on enteroendocrine L cells induces secretion of GLP-1, a key modulator of insulin synthesis and secretion, (15)(18) and cholecystokinin (CCK), a satiety hormone known to slow gastric emptying and increase dietary fat absorption.(61) Furthermore, bitter tastants have also been shown to reduce gastric motility.(62) Genetic variation in taste receptors may alter the chemosensory signals that stimulate the cephalic phase of salivary, gastric, pancreatic and intestinal secretions.(63)(64)(65)(66)
Taste sensations, previous dietary experience and hunger modulate cephalic phase salivary responses.(67)(68)(69) The chemosensory cephalic phase exocrine and endocrine responses include secretion of trypsin, lipase, chymotrypsin, pancreatic polypeptide, somatostatin, neurotensin, CCK, GLP-1, insulin and ghrelin, among others, which together modulate appetite, influence the efficiency of nutrient absorption and regulate meal size.(4)(70)(71)(65)In regulating appetite and satiety, GLP-1 works both peripherally and centrally in concert with leptin, a product of adipose tissue and inhibitor of appetite. Human studies demonstrate that these peptide hormones are indeed significantly associated with eating behaviors(72)(73) providing a route through which variation in taste receptors could impact upon eating behaviors.
Eating behavior is a heritable trait.
Fat Taste
Whether fat elicits a distinct taste perception is controversial, but evidence supporting the ability of at least some taste cells to detect fatty acids (FAs) is compelling. However, the identity of any FA-sensitive taste receptors remains unclear. The FA transporter CD36, a member of a family of proteins expressed both within lysosomes and at the cell surface that function in selective cholesterol uptake from high-density lipoproteins, is necessary for behavioral preferences for FAs in mice.(74) CD36 is expressed in taste cells, intestines, adipose and other tissue with high FA flux or utilization.(75) FA interaction with CD36 contributes to transducing signals that alter lipid utilization downstream, including secretion of adipokines such as leptin and adiponectin, which play an important role in the regulation of energy expenditure from lipids. Genetic variation in CD36 has been associated with increased risk of cardiovascular disease(76) and type 2 diabetes mellitus,(77) however no direct links have yet been made with dietary fat intake or eating behavior.
Summary
The sensory experience of food is a primary reinforcer of intake. Individuals eat what they like and avoid what they dislike, impacting food intake and diet-related chronic diseases. Both genetic and environmental factors influence food preference, intake and eating behavior. Ample evidence suggests that genetic variation in taste receptors, particularly TAS2Rs, influences ingestive behavior and risk for chronic disease. Of the GPCRs transducing taste, TAS2R38 is best characterized for its role in taste and influence on eating behavior. However, clinical screening for PROP taste sensitivity does not predict those at risk for obesity or increased BMI.(78) The influence of genetic variation in taste receptors on eating behavior and the control of food intake varies, and some individuals may be more susceptible to these genetic influences than others.