Course Authors
Susan C. Stewart, M.D.
Dr. Stewart reports no commercial conflicts of interest.
This activity is made possible an unrestricted educational grant from ![]() .
.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the relationship between human papillomavirus (HPV) infection and the development of cervical cancer
Describe the natural history of cervical HPV infections in women
Discuss the new thinking on Pap smear interpretation that reflects either transient or persistent HPV infection
Describe test types and testing procedures for HPV infection
Summarize current state of knowledge about HPV vaccines.
In 1943 George Papanicolaou published his monograph that became the basis of the Pap test. During a period of 35 years from 1947 to 1982, among white American women, the Pap smear decreased the incidence of cervical cancer by 75% (32.6 to 8.3/ 100,000) and the death rate from cervical cancer by 80% (10 to 2/100,000).(1) Now, among all U.S. women, cervical cancer has dropped to just over 10,000 new cases and 3,700 deaths per year.
Screening rates are very high, around 80%(2) This is a cancer that can be prevented by detection of premalignant cellular changes followed by timely treatment.
The news is not so good in the developing world. With minimal infrastructure to do cervical cancer screening, much less evaluate abnormalities and treat lesions, cervical cancer rates remain in the pre-1940s range. In many developing countries invasive cervical cancer is the most common cancer in women. Worldwide there are 470,000 new cases and 230,000 deaths annually.(3)
Now research on the role of human papillomavirus (HPV) in cervical cancer development, HPV testing procedures and, most recently, a vaccine against the virus may change these discouraging statistics.
Human Papillomavirus in the Genital Tract
In the last 30 years there has been a revolution in our knowledge about the causation of cervical cancer as papers on the association between human papillomavirus (HPV) and cervical cancer began to appear (1975). Then a 1983 publication described papillomavirus DNA in cervical cancer cells and later the connection was made between HPV type 16 and the risk of cervical cancer (mid 1990a). Now a number of HPV types can be classified as high risk for cervical cancer.(4),(5)
There are more than 100 types of papillomaviruses that affect humans. The most familiar ones are those that cause skin warts. Over 40 types affect the genital tract, some causing genital warts (condyloma accuminata). The best known genital-wart-causing types are 6 and 11 and are considered low-risk for cervical cancer. Other types cause infections of the cervix, affecting the transformational zone between the squamous epithelium of the vagina and cervix, and the glandular epithelium of the cervical canal.
In 1995 types 16 and 18 were classified as human carcinogens(7) and in the U.S. are found in association with 70% of cervical cancers, type 16 being associated with 60%. The DNA test for HPV incorporates 13 high-risk types of the 18 types considered high-risk.(5) High-risk types show different distribution patterns in different parts of the world, factors that have to be considered when testing women for persistent high-risk infections.(8) HPV is considered an initiator of cervical neoplasia. Other influences such as cigarette smoking, nutrition and Chlamydia infection are considered promoters of cervical neoplasia.
The Natural History of Genital HPV in Women
Genital HPV is a sexually transmitted infection. Most sexually active young women will become infected, 40% within 16 months of first vaginal intercourse.(21) The infections can be with different HPV types and more than one type at a time. The infection is usually clinically inapparent. It may remain dormant for months or years before becoming detectable. Most infections are transient, clearing completely within 9-15 months, as immunity develops.
A small number of infections, five to ten percent, become persistent, and it is from these that the problems arise. In these cases, the virus becomes incorporated into the genome of the cervical cells and viral oncogenes become active.(6) The cells develop changes that are picked up by Pap smears or biopsy. These changes are called intraepithelial because they are confined to the epithelial layer. They can be seen in both the squamous and glandular epithelium. Down the line, either a squamous or adenocarcinoma could develop. Squamous is much more common than adenocarcinomas, which make up less than 20% of cervical cancers. In developed countries there is an increase in the proportion of adenocarcinoma of the cervix.(9)
The peak prevalence of female genital HPV infection is in the third decade (20s). As time goes by, immunity builds and sexual habits become more conservative. So with the 10-15 year latent period, women infected in their 20s who develop persistent infection would not progress to cervical cancer until their late 30s or 40s. So as we see a rapid decline in HPV infection in the late 20s and 30s, we see a slow rise in the incidence of cervical cancer in the 30s and 40s. As women acquire and maintain a persistent high-risk HPV infection, and other cofactors or promoters of neoplasia become active, they are at high risk for cervical cancer.
Cervical Cancer Risk Factors and HPV
Though the years, long before the HPV role was known, a number of risk factors for cervical cancer development were identified. Now that we know about HPV the reasons for these factors become clearer:(9)
- Very early age of onset of sexual activity (<16 years old): This probably has to do with the extreme vulnerability to HPV infection of the transformational zone in the adolescent.
- Multiple sex partners (more than four): There will be more opportunities to contract more types of HPV.
- Cigarette smoking: There are carcinogens in cigarette smoke that have been identified in the cervical mucus and promote malignant change. This is operative in squamous but not in adenocarcinoma.(9)
- Decreased immunity: Women receiving immunosuppressive drugs and now those with HIV/AIDS have an increased risk for abnormal Pap smears and cervical cancer. These patients have a poor immune response to HPV. Specific defects in immunity not related to a clinical diagnosis may be operative in women who develop persistent infections with HPV.
In our efforts to find cases of cervical cancer we have taken this high-risk history into account, made extra efforts to follow up these patients or instituted more stringent screening protocols in facilities with large percentages of patients with more risk factors. Now that we know about the role of HPV and have tests to detect HPV, we can further narrow our scrutiny by identifying patients with persistent HPV infection. After all, a woman could have many sexual partners, and many HPV infections, but if she develops robust immunity to HPV and never has a persistent HPV infection she is a very low risk to get cervical cancer.
We have careful constructs for screening and following up patients with abnormal findings but one risk factor we always have to keep in mind is the problem of women who do not return for follow up. The best-laid algorithm does no good if the patient does not return for the next visit. The more we can narrow the population for vigorous follow up by using HPV testing, the more time we will have to pursue the highest risk patient.
The Tests for HPV
What tests have been developed for HPV? What tests do we use for HPV? As you can guess from the preceding discussion on natural history, we now have type-specific tests for HPV with serum antibodies. Although this test tells us that the patient has had an infection with a specific HPV type, it does not tell us if she is currently infected with high levels of the virus, a circumstance needed for neoplastic changes. In order to know that, you have to test vaginal or cervical secretions directly for the viral DNA.
One group of tests is based on hybridization techniques. HPV is a double-stranded DNA virus. In hybridization, an RNA probe seeks the DNA and the resulting "hybrid" of DNA-RNA is detected, using such techniques as electrophoretic separation or radioactive labeling. Another technique is polymerase chain reaction, or PCR amplification of the HPV DNA. This procedure can detect extremely minute amounts of type-specific DNA and, though popular in research, is not used clinically at this time.
The current FDA-approved HPV test is based on hybridization. The DNA-RNA hybrids are "captured" on a microplate coated with antibodies that are specific to the DNA-RNA hybrids. The technique has been named "Hybrid CaptureRx." These hybrids are reacted with antibodies, one of which produces chemiluminescence. The actual amount of viral DNA present is measured by a device that detects luminescence, relative light units (RLUs) from the hybrid compounds. This test, however, is not type-specific and detects several HPV types. Although various panels of viral types are available for testing, the high-risk panel, detecting types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59 and 68, is the relevant test to use to look for persistent high-risk HPV infection. In 2003 the FDA approved this test, known as the Hybrid CaptureRx 2 High Risk HPV DNA TestTM (Digene) for women 30 and over to use adjunctively with the Pap test in primary screening. The test is also approved to triage patients with certain types of abnormal Pap smears and to help decide whether to do colposcopy. (See www.fda.gov, www.digene.com, www.asccp.org)(15)
Testing for HPV: Does this Patient have Chronic Infection?
As I mentioned in the discussion on natural history of genital HPV, the infection is common in young women up to their mid-twenties. Most infections, whether with high-risk or low-risk viruses, are cleared by nine to 15 months. What we want to know is whether the patient has a chronic infection with high-risk HPV. In other words, what sets off the changes that can lead to cervical cancer? We can use HPV testing after the 20s to look for persistent infection.
The Pap Smear Today
The Pap smear is still the central player in cervical cancer detection. There is new technology. There have been changes in terminology for abnormal Paps, which I have reviewed before and must again because they were revised in 2001. There are changes in the guidelines on Pap smear screening and the triage of abnormal Paps, and HPV testing has been incorporated into both of these guidelines.
Changes in Pap Technology
We think of the Pap test as the examination of a slide prepared at the time of pelvic exam. This is known as conventional cervical cytology. The technique has the disadvantage of having to be done quickly while the pelvic exam is still in process and may result in thick slides that are hard to read. The thin, wet or liquid prep is a new technology wherein the cervical sample is placed into a liquid fixative. The slides are prepared in the lab later, manually or by machine. The main advantage of the liquid prep is the ability to spread the cellular material thinly and evenly over the slide so that the cells are clearly visible and not obscured by mucus or other cellular debris. A second advantage is that the liquid sample can be tested for HPV, so that the patient does not have to return for another vaginal or cervical sample for that test.
Is one technique better than the other? In its screening guidelines, the American Cancer Society has allowed for screening Paps every two years if the liquid-based cytology is used. The American College of Obstetrics and Gynecology (ACOG) still recommends every year with either test.(11) Although a higher sensitivity and specificity have been reported for the liquid-based prep in many studies, a recent comprehensive review of all studies casts some doubt on the superiority of the liquid prep and calls for randomized controlled studies.(7)
Changes in Pap Terminology: the Bethesda System and Others
The Bethesda System has tried to simplify and universalize cervical cytology nomenclature but we all have a memory for old systems.

Table 1. Comparison of Nomenclature Used for Pap Smears and Cervical Biopsies.
| Bethesda Syst, IL: intraepithelial lesion | LSIL: low-grade intraepithelial lesion | HSIL: high-grade intraepithelial lesion | ||
| Atypia | Mild, HPV changes | Moderate | Severe | |
| Dysplasia Papanicolaou | II | III | IV | V |
| CIN: cervical intraepithelial Neoplasia/ CIS: carcinoma in situ |
CIN-1 | CIN-2 | CIN-3 | CIS |
| Depth of mucosa involved | Basal 1/3 | Basal 2/3 | >2/3 | full thickness |

In the original Pap terminology, various gradations of dysplasia were recognized, from Mild to Moderate to Severe. Now Mild is identified with LSIL, low-grade squamous intraepithelial lesion and Moderate and Severe with HSIL, high-grade squamous intraepithelial lesion. The term "intraepithelial lesion" denotes the correlation between the exfoliated cells and the degree of epithelial involvement on a biopsy specimen. A more dysplastic lesion involves a greater thickness of epithelium, starting with the basal layer.
Another system uses the term "cervical intraepithelial neoplasia" (CIN) and is applied to biopsy findings. CIN 1 involves the basal one-third, CIN 2 involves up to two-thirds and CIN 3 involves more than two-thirds of the epithelium. Carcinoma in situ, CIS, has full thickness involvement without penetration of the basement membrane. CIN 1 is associated with LSIL and CIN 2 & 3 and CIS with HSIL.(12),(13)
The Bethesda System and a New Category for the Pap
A smear from an epithelium that is evolving toward malignancy may contain a variety of cells. You may not see the severely dysplastic cells that clinch the diagnosis of HSIL but less abnormal cells that may raise a suspicion. These cells may be similar to those with changes induced by inflammation or infection. For these types of cells the 1991 Bethesda System introduced the term "ASCUS" (Atypical Squamous Cells of Undetermined Significance) to describe equivocal cells that need follow up.
In the 2001 Bethesda revision, an effort was made to increase the precision by calling the whole category ASC (Atypical Squamous Cells) and subdividing it into ASC-US (Atypical Squamous Cells of Undetermined Significance) and ASC-H (Atypical Squamous Cells, cannot exclude HSIL). The old term "ASCUS, favor reactive," was dropped, and pathologists were encouraged to call those changes "negative for intraepithelial lesion or malignancy."(13)
For most of this Cyberounds® I have discussed cervical cancer of squamous cell origin. There is also adenocarcinoma of the cervix and it is becoming a larger proportion of cervical cancers in developed countries. Adenocarcinoma of the cervix arises from the glandular cells of the endocervix and is often hidden in the cervical canal. The category Atypical Glandular Cells, ACG is similar to ASC in that it also must be followed up thoroughly. It can also be associated with HPV infection and with squamous cell neoplasia.
Papanicolaou Tests and Neoplasia Detection in Perspective
About 50 million Pap tests are done annually in the United States. Of these, around 5% demonstrate neoplastic dysplasia. On average, ASC is 3-5%; LSIL, 1.6%; and HSIL, 0.45%. [9,13] The goal of triaging these Paps is to detect and treat CIN 2 & 3 and higher lesions (CIS and invasive cancer). The triage guidelines try to achieve this goal by taking into account risk and avoiding the expense, inconvenience and anxiety of advanced procedures like colposcopy. At the same time, they need to incorporate a margin of safety so that the fewest possible serious lesions will be missed.

Figure 1. Normal and Abnormal Pap Smear.
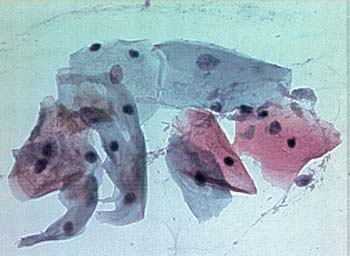
Panel A. Normal Squamous and Transitional Cells: large amount of clear cytoplasm, small regular nuclei.
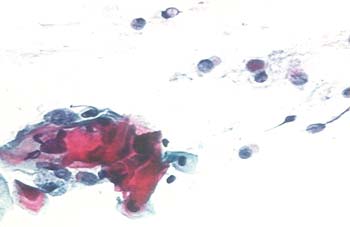
Panel B. HSIL (CIN-2): decreased amount of cytoplasm, compared to nuclei; enlarged, irregular nuclei; coarse chromatin pattern.

Where does HPV Testing Fit in Following Up Abnormal Paps?
The revelation of the role of HPV and its natural history in the female genital tract have helped to correlate the changes caused by the infection with the changes long observed on Pap smears. It seems that ASCUS and LSIL (CIN1), changes that frequently revert to normal on subsequent testing, are most often associated with the transient HPV infections. The progression to HSIL (CIN 2, CIN 3 and CIS) is associated with the persistent infections.
HPV Testing and Management of Women with Cervical Cytological Abnormalities
It is not my purpose to review the spectrum of management of abnormal Paps. I want to point out where HPV testing has been inserted to improve our ability to find advanced lesions. There is an excellent review of management(14) and several sources of algorithms that lay out the schemes of initial and advanced management that you can consult. For a complete and very readable guideline on all aspects of cervical screening, go to the ASCCP website (www.asccp.org).(17)
HSIL and HPV
There is no point in HPV testing patients with HSIL. They have to be colposcoped and biopsied anyway.
ASC-US and HPV
HPV testing is now another alternative for ASC-US Paps. You now have three alternatives: you can repeat the Pap at 6 and 12 months, go straight to colposcopy or get the HPV DNA test for high risk HPV types. If HPV is positive, proceed to colposcopy and biopsy. If negative, resume the normal screening schedule. A negative HPV test provides reassurance that the ASC-US is not signaling neoplasia and yearly Pap testing can be continued. When the liquid prep is used, HPV can be done on the same specimen and a new sample does not have to be obtained. Often a "reflex test" is ordered, so that HPV is automatically done, based on the cytology.(14)
LSIL and HPV
Although initially it was thought that there was a steady march from mild to severe dysplasia, or LSIL to HSIL, we now find that LSIL may be a manifestation of HPV infection, as is ASC-US in many cases. While most women with LSIL are referred for colposcopy, a more conservative approach has been adopted in adolescents and young women, in whom the cytological changes may be a manifestation of a transient infection. The cytology can be repeated at 6 and 12 months and, if negative twice, the patient goes back to yearly screening. At 12 months, high-risk HPV testing is done to look for persistent infection. If high-risk HPV DNA is positive at 12 months, the patient is referred for colposcopy. If HPV is negative, she goes back to yearly screening.(14)
A study of regression of LSIL in young women found that infection with HPV at the time of the initial cytology of LSIL had no effect on regression rate, implying that taking the test at that time would have little bearing on management. The researchers also noted an association of infection with multiple types of HPV (not necessarily high-risk) with persistence of LSIL. The authors suggest that an immune defect may be operative in these cases.(17)
ASC-H
The 2001 Consensus guidelines recommend colposcopy in all cases, although some authorities now feel that ASC-H can be treated like LSIL in young women.(15),(17)
HPV Testing After Colposcopy
With LSIL and ASC-H, when colposcopy is negative or shows CIN 1 on biopsy, an HPV test at 12 months is recommended. This is to determine whether persistent HPV is present and, if so, another colposcopy should be done.
If you want more details on following up abnormal Paps, consult the guidelines on the ASCCP website.
Primary Screening Guidelines for Cervical Cancer
When the Pap Test Was Our Only Screening Tool
If you think about the procedure of the Pap smear, you can imagine that cells may be missed, particularly in the early stages of dysplastic changes when the abnormal cells are closest to the basement membrane of the epithelium. Other problems such as bleeding and inflammatory cells can interfere with the detection of neoplasia. If the quality of the cervical sample is poor, because of interfering cells or inadequate numbers of cells from the transformational zone, the test must be redone. Furthermore, the technician does not view every cell on the slide but rather a sampling of the cells. The bottom line is that the Pap is considered rather insensitive, i.e., it can have a high number of false negatives.

Sensitivity = |
TP (true positives) TP + FN (false negatives) |

To make up for the lower sensitivity, we have recommended screening yearly to reduce the effect of false negatives, considering that, if it is a progressive lesion, it will be revealed by the next Pap smear. HPV DNA testing is more sensitive than Paps, so it complements Pap testing and alerts us to chronic infection and the need for more frequent follow up.
How to Add HPV Testing to Primary Screening
"Primary screening" refers to the population of women going for a routine Pap smear. They have no symptoms and they are not receiving surveillance for a previous abnormal smear. Thinking about the natural history of HPV, there is absolutely no point in doing a high-risk HPV DNA test in young women. Many of them will be positive with high-risk types and most will not develop chronic infection. If you listen closely to the Direct-to-Consumer ads about HPV testing, you will note that the very end of the presentation, the announcer says the test is for "women over 30." The FDA says this is a test to be done adjunctively with the Pap smear. The goal is to pick up chronic high-risk HPV infection even when the Pap is negative in a woman 30 and over.
So here are the highlights of the American Cancer Society Guidelines(11):
- First screening Pap: three years after first vaginal intercourse or age 21, whichever comes first.
- Until age 30: Pap every year with conventional smears or every 2 years with liquid prep. (ACOG says every year with either test)
- After 30: If the woman has had three consecutive negative Paps, she may have screening every two or three years using either conventional or liquid-based cytology.
Alternatively, she may have screening every three years with conventional or liquid-based cytology AND high-risk HPV DNA test.
Negative cytology and negative high-risk HPV test have a very high negative predictive value for cervical intraepithelial lesion and provide reassurance that screening every 3 years is safe. The likelihood of HSIL at 3 years is 1 or 2/1000 (0.01-0.02%).

Negative Predictive Value = |
TN (true negatives) TN + FN (false negatives) |

When cytology is negative but high-risk HPV is positive, the likelihood for cervical intraepithelial neoplasia in the next three years is 4.3%. Resuming yearly Pap screening is recommended to pick up any neoplasia that may develop.(16)
A Vaccine against Human Papilloma Virus
The vaccines are based on a technique described in 1991, when papillomavirus-like particles (VPLs) were developed in the laboratory. These empty shells contained no viral DNA so could not be infectious. These shells were composed of L1 proteins specific to HPV types and elicited a brisk immune response and protection against infection with that type.(18) At the beginning of this decade, papers began to appear about the L1 VLP (virus like particle) vaccines against HPV types high-risk for cervical cancer. In 2002 a paper by Koutsky described a group of young women without evidence of HPV 16 infection serologically or vaginally, who were 100% protected from persistent HPV 16 infection and cervical intraepithelial neoplasia by a vaccine against type 16. The vaccine was given in 3 doses, at 0, 2 and 6 months. It was highly immunogenic, producing antibody levels almost sixty times that of natural infection.(20)
Now phase II studies have been reported on a bivalent vaccine containing types 16 and 18(3) and a quadrivalent vaccine containing types 16, 18, 11 and 6.(19) Again the studies showed very high titers of antibodies and protection against chronic infection and intraepithelial lesions. Expanding the number of types is important because many types are high-risk for cervical cancer, although types 16 and 18 account for about 70% of cervical cancers. Type 16 is the most common and is typically associated with squamous cell cervical cancer, while type 18 is more often associated with adenocarcinoma.
Because dysplastic glandular epithelial lesions may be located in the cervical canal, the precursor lesions of adenocarcinoma are harder to detect by Pap smears.(4) As I noted before, the proportion of adenocarcinomas of the cervix is increasing in developed countries. These oncogenic HPV types are not just associated with cervical cancer. They are seen in cancers of the vulva, vagina, penis and anus. Types 11 and 6 are associated with genital warts in both sexes and with oropharyngeal infections such as recurrent respiratory papillomatosis, a rare and difficult to treat infection in children and adults.(4)
In December 2005 one pharmaceutical company sent the FDA a submission for the quadrivalent vaccine (types 16, 18, 11 and 6). Sometime in 2006 a second pharmaceutical company indicates it will submit an application for the bivalent vaccine (types 16 & 18).(21)
How will these vaccines fit into the fight against cervical cancer? These vaccines are geared for individuals without previous HPV infection and must be given prior to the initiation of sexual activity. After sexual activity begins, 40% acquire HPV within 16 months.(21) In developed countries with a strong health infrastructure, a three-shot program could be inserted into the pediatric vaccine schedule. Presently we don't know how long immunity lasts. Would booster shots be required? Should both girls and boys be immunized? After all, the virus is transmitted by the male and immunization will further decrease the prevalence of high-risk types. Besides, males can develop cancers and genital warts.
In resource-poor countries where cervical cancer is the first or second cause of cancer death in women, a vaccine that would protect a woman against cervical cancer for life would dramatically decrease mortality from cervical cancer. There are many hurdles before such a goal could be achieved but we are well on our way.
Finding the Infection, Preventing the Cancer
It is exciting to think of a phalanx of immune young women marching through the age cohorts until cervical cancer all over the world is as low as in developed countries. What about developing countries where facilities and equipment are sparsely distributed and multiple visits are very hard to achieve? These countries have many chronically infected women who are moving through the stages of oncogenesis with no intervention for lack of resources.
With all the knowledge about the role of HPV, new approaches are being proposed. Cost-effective analyses using data from five developing countries suggested that screening at age 35 with visual inspection of the cervix or HPV DNA testing could reduce lifetime risk of cervical cancer by close to 30% and that screening at age 35 AND 40 would reduce lifetime risk by an additional 40%.(23)
Another author proposed an overall scheme that would include an initial vaccination and subsequent screening for high-risk HPV at ages 35 and 40. A one-shot vaccine containing the high-risk types prevalent in the region would be needed. Then scarce resources would be concentrated on finding and treating neoplasia in women with persistent infection.(21)
Finally, maybe it is time to rethink cervical cancer prevention altogether. Rather than morphology-based, using the relatively insensitive but highly specific Pap smear, maybe it should be based on a test for the sexually transmitted infection, the high-risk HPV, which is very sensitive after age 30. One author has likened this to the approach to syphilis, using a highly sensitive screening test, the VDRL or RPR, and triaging that with a highly specific one, the FTA-ABS.(24)
Summary
We now know that HPV is the initiator of cervical cancer in almost every case. We now have tools to screen women for persistent infections with high-risk types of HPV and then detect and treat the precancerous cellular changes. We now also have vaccines that can prevent persistent HPV infections. As a result of these advances, there is great potential to further decrease cervical cancer in developed countries and to dramatically lower the incidence and mortality for cervical cancer throughout the world.