Course Authors
Annie-Claire Nadeau-Fredette, M.D., and Joanne M. Bargman, M.D.; Eduardo Alas, M.D., and Mark Unruh, M.D., M.S.
Dr. Nadeau-Fredette is Home Dialysis Fellow, The Home Peritoneal Dialysis Program, University Health Network, and Dr. Bargman is Professor of Medicine, University of Toronto, Toronto, Canada; Dr. Alas is Assistant Professor, and Dr. Unruh is Solomon, Gardner & Sterling Chair, Chief of Nephrology, Department of Internal Medicine, University of New Mexico School of Medicine, Albuquerque, NM.
Within the past 12 months, Drs. Alas and Nadeau-Fredette report no commercial conflict of interest; Dr. Bargman has been a consultant to Amgen, been on the Speaker’s Bureau for Amgen and DaVita Healthcare, and received grant/research support from Baxter Healthcare; Dr. Unruh has received grant/research support from Dialysis Clinic, Inc.
Albert Einstein College of Medicine, CCME staff, and interMDnet staff have nothing to disclose.
This activity is certified for nephrology, primary care (internal medicine and family practice), pediatrics, and for all HCPs interested in the treatment of patients with chronic kidney disease. This CME activity has been peer-reviewed by Ladan Golestaneh, M.D., M.S., Associate Professor of Medicine, Renal Division, Albert Einstein College of Medicine Montefiore Medical Center, Bronx, New York
This activity is made possible by an unrestricted educational grant from Baxter International Inc.

Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Identify ESRD patients who may benefit from home hemodialysis (HHD) or home peritoneal dialysis (PD)
Discuss and apply clinical guidelines for home-based dialysis
Manage any comorbidities (e.g., ?nutritional status, frailty, ?physical fitness) associated with ESRD and apply clinical measurements to assess patient response to HHD/PD
Identify barriers (e.g., deficient housing accommodations) to HHD/PD as well as possible remedies/adjustments.
This presentation may include discussion of commercial products and services.
The prevalence of renal disease in the United States continues to increase dramatically. It is estimated that one in ten Americans have some level of chronic kidney disease (CKD). This represents more than 20 million patients in the United States with this ailment.
The largest (and expanding) group of CKD patients are patients 65 years of age and older (see Figure 1). Only a minority of these patients proceed to hemodialysis (HD).

Figure 1. Proportion of Chronic Kidney Disease By Patient Age.

The High Cost of End Stage Renal Disease
In 2010, approximately 600,000 patients carried the diagnosis of end stage renal disease (ESRD). While, in 2008, the average Medicare patient expenditure per patient was $10,188, in that same year the average Medicare expenditure for the ESRD patient was $65,256. This $65,256 average cost is in sharp contrast to the $9,676 spent on the same Medicare per age beneficiary (65 years and older) who does not have ESRD.
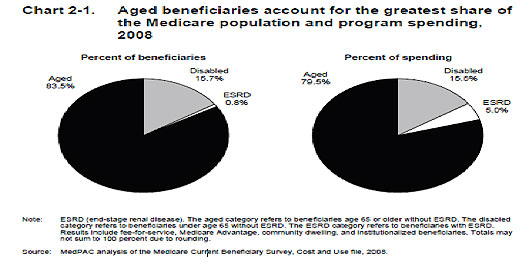
Medicare spending on ESRD patients is clearly unbalanced. It is estimated that 0.8% of the Medicare beneficiaries require 5.0% of the total Medicare spending (Figure 2). The cost of in-center dialysis is high and the mortality of a dialysis patient is also high. Approximately 90,000 patients die per year while on dialysis.(1) In 2008 the average expected lifespan of all patients on dialysis was 7.1 years.(2)

Figure 2. Medicare Spending By Program In 2008.


We anticipate continued growth in the number of patients undergoing dialysis worldwide due to the aging of the population and the increasing prevalence of diabetes. With a larger cohort of people undergoing dialysis, increases in secondary medical ailments specifically related to in-center dialysis will occur. As an example, infection control and cardiovascular disease will become challenges to maintaining in-center hemodialysis populations.
A Rising Rate of Infection
An increased rate of infection, for example, can be expected with more dialysis patients. The rate of hospitalization for bacteremia or septicemia among maintenance hemodialysis patients in 2010 was 116 events per 1000 patient-years.(3) This was a 51% increase compared to 2002 when there were fewer patients on dialysis.
Another example of infectious risk for in-center hemodialysis is the high rate of hepatitis C (HCV). A patient on chronic in-center hemodialysis is five times more likely to have HCV than those in the general population,(4) and there have been documented cases of transmission of HCV among in-center hemodialysis patients.
Even though the risk of transmission of HCV while undergoing in-center hemodialysis is low and can be addressed by aggressive infection control and surveillance, home hemodialysis (HHD) may further reduce risk of transmission.
Given the high costs and high morbidity/mortality of in-center hemodialysis, home hemodialysis may represent an alternative approach to in-center hemodialysis. Home dialysis solutions have the potential to mitigate the rise in these dialysis-associated complications, hence the renewed focus on taking the dialysis patient back to his/her home.
Potential Benefits
Patients may benefit both in terms of quality of life and improved dialysis metrics by performing home hemodialysis (HHD). The ability to dialyze at home and control one's own health have been reported as major reasons for deciding to perform HHD.(5)
Setting Your Own Schedule
Home-based dialysis allows patients to set their own treatment schedule. This may make it easier for patients to meet the demands of work or school or attend to personal needs. It may also help relieve some of the problems related to sleep. For example, a patient with restless legs syndrome (RLS) on an afternoon or evening shift may have difficulty tolerating a full hemodialysis treatment; however, this patient with RLS may have a better experience earlier in the morning.
Travel Time
Another important advantage to home treatments in our practice is reducing the need to travel to the dialysis unit thrice-weekly. In isolated rural areas, it can take several hours of travel time per treatment to attend in-center dialysis. We have transferred a number of patients to either home hemodialysis or home peritoneal dialysis for this reason alone.
Reduced Ultrafiltration Rate
Home hemodialysis permits patients to adjust the treatment frequency, timing and duration to account for changes in volume status. Both short-frequent treatments and longer treatment times reduce the ultrafiltration rate. A lower ultrafiltration rate has been associated with improved cardiac outcomes and survival.(6)(7)(*) The ability to personalize the timing of treatments can be limited in the HD units where patients have a rigid schedule for on and off treatment in order to maintain workflow and promote safety.
Improved Serum Phosphate
Both more frequent treatments and long-slow home hemodialysis may additionally improve serum phosphate and nutritional status.(9) Managing hyperphosphatemia in some patients with dietary interventions and phosphate binders can be difficult. In this subgroup of patients, home hemodialysis may permit a more flexible dialysis, which may better control hyperphosphatemia.
Higher Patient Satisfaction
HHD may also improve patient well being and patient satisfaction with care. The Frequent Hemodialysis Network Study examined whether home nocturnal dialysis significantly improved cardiac outcomes and quality of life compared to a group largely composed of home thrice-weekly dialysis.(10)(11)(12)
The Nocturnal Study of the Frequent Hemodialysis Network Study did not demonstrate a significant benefit in health-related quality of life outcomes compared to thrice-weekly home dialysis.(11) One could interpret this finding that the use of nocturnal dialysis did not have a significant effect on patients' well being. However, it is possible that the comparator group of thrice-weekly home hemodialysis had improved health-related quality of life, making it difficult for the nocturnal arm to show a substantial improvement. The higher health-related quality of life scores of the home thrice-weekly group compared to the in-center thrice-weekly group support this premise.
The FREEDOM Study followed a cohort of patients undergoing frequent home dialysis using the NxStage machine over the course of one year. The findings from the FREEDOM Study demonstrated improvements in restless legs symptoms, depressive symptoms and overall quality of life after patients transitioned to frequent home treatments.(13)(14)(15) However, many patients dropped out of the FREEDOM Study which could bias the outcome assessment.(16)
Home hemodialysis may enhance patient satisfaction with care. Several studies have demonstrated improved patient satisfaction with peritoneal dialysis compared to home dialysis.(5)(19) Patients have reported greater satisfaction with HHD compared to in-center dialysis.(5) Hence, HHD has the potential to improve both health-related quality of life and patient satisfaction among the ESRD population.
Clinical Guidelines for Home Hemodialysis
Although dialysis at home is not a new concept, guidelines for this "revived" technique have not been standardized. The main clinical concern for all clinicians is whether their dialysis patient is receiving adequate dialysis. Urea clearance is the standard by which all in-center HD patients are assessed. The minimum standard is expressed as Kt/V <, a dimensionless ratio where K represents dialyzer clearance, t is dialysis time, and V equals volume of distribution of urea—approximately the patient's total body water.
The minimum standard for which mortality is not increased was initially determined by the Hemodialysis Study (HEMO) group.(44) That Kt/V is greater than 1.3, and is now the Kidney Disease Outcomes Quality Initiative (KDOQI) minimal adequacy standard; however in home dialysis patients this number has yet to be determined. Increased frequency, such as may be the case with some HHD patients, increases efficiency, while the current KDOQI recommendation relies instead on a continuous equivalent clearance (Kce). This clearance is noted to be higher than consensus-derived Kce for PD. A consensus-derived clearance adequacy has yet to be determined for HHD, but likely is forthcoming as regulatory agencies have taken notice.
Equipping the Home for Dialysis
Home dialysis entails using one of two types of modalities, hemodialysis or peritoneal dialysis machines. Peritoneal dialysis is an option that has been offered for many years in the United States via a manual exchange system of peritoneal fluid that only involves a clean room, a stand and dialysate bags. Peritoneal dialysis is further discussed in detail below.
Home hemodialysis is more elaborate but, potentially, offers greater benefit because it reduces the amount of time patients need to be on dialysis and provides for a more "programmable" amount of fluid that may be removed.
There are two widely used options to provide HHD. One, a small machine the size of a tabletop that weighs approximately 90 pounds, is capable of regenerating dialysate from any potable water source. A simple under the sink connection to a home water faucet and a standard electrical outlet are all that are necessary. It is capable of going "on the road" via bagged dialysate fluid for the patient that enjoys mobility and travel.
The second machine option is a version of an in-center hemodialysis machine and weighs approximately 160 pounds. It requires an additional reverse osmosis device to function and is not as portable. This machine does not have the capacity without additional components to convert home water into dialysate. However, this machine allows for greater control of dialysis because it is able to use a variety of current dialyzers that target uremic removal.
Remote monitoring and the ability to administer heparin in a maintenance mode so that clotting of the access is avoided are also available. Several alternative platforms to home hemodialysis may be entering the market over the next few years to address the limitations of portability and prescription flexibility that the bigger home dialysis machine is able to provide.
How the Machines Work
Regardless of which HHD system a patient choses, the blood is still accessed via an arterial venous fistula that is created by a surgeon prior to initiation of hemodialysis and allowed to mature, usually over a 3-month period. The fistula is accessed for dialysis by placing two needles into it in a sterile fashion by the patient or caretaker. One needle is to withdraw "dirty" blood and the other is to return the "cleaned" blood to the patient. It can often be a source of high stress for patient to access their fistula, especially given that many patients on dialysis have some form of visual impairment.
Patient Training
Home dialysis therapies involve many hours of nurse teaching so that patients may be proficient and comfortable with managing their own care. It begins by assessing patient compliance and progresses to visiting the patient at home to evaluate the appropriateness of a home treatment room.
Patients are shown the best way to access their fistula and are encouraged to "button hole," a process by which the patient uses the same needle insertion site, so that it may epithelialize, to facilitate future fistula access. Patients are also taught how to maintain dialysis machines, treatment logs, and record blood pressures. Patients are expected to be confident in their skills to recognize and report any problems to a nurse who is on call and available to them 24/7.
Obstacles to Home Hemodialysis
The limitations of training, bleeding and burnout may contribute to the very slow adoption of home hemodialysis in the United States. HHD patients and caregivers are trained to assess the vascular access, blood pressure, fluid removal, water quality and dialysis machine alarms.
This intensive treatment regimen can take several weeks and requires nurse educators and staff to support the patient and family. It is interesting that formal level of education and cognitive performance were not associated with the time required for HHD training.(17)
Home hemodialysis may have a mixed impact on the psychosocial well being of patients.(18) Some patients are unable to perform HHD due to anxiety and depression. Patients and caregivers undergoing HHD may develop fatigue from the burden of having to perform near daily dialysis treatment. While burnout has been anecdotally observed, there are little data on the rate of burnout to HHD regimens or predictors of HHD burnout.
Summary
Home hemodialysis provides the opportunity for ESRD patients to provide self-care in a familiar setting. The limitations of training, bleeding and burnout should be addressed so that more patients may have access to this mode of renal replacement therapy. Its benefits—lower costs, better quality of life and improved physiologic and psychological well being—should motivate providers to establish home programs. HHD may be particularly useful in areas with limited access to dialysis centers such as rural and urban areas.
Although used by a small proportion of dialysis patients in the United States, home peritoneal dialysis is associated with a better quality of life than conventional in-center hemodialysis (HD) with a similar survival rate.(20)(21)(22)(23) With the exception of initial training sessions and acute dialysis, peritoneal dialysis is practically always performed in a home setting. For this reason, the term peritoneal dialysis (PD) rather than home peritoneal dialysis will be used in this review as it is in the PD literature. However, the reader should always keep in mind the intrinsic 'home' nature of PD.
There are two major prescription patterns in PD, which can all be performed in a home setting. Continuous ambulatory peritoneal dialysis (CAPD) refers to manual exchanges of dialysis bags, on average four times per 24 hours. Automated peritoneal dialysis (APD) is usually performed by a cycler machine with three to four exchanges overnight, and a day exchange or, less frequently, with an empty abdomen during the day in nocturnal intermittent peritoneal dialysis (NIPD).
Who Can Benefit From Peritoneal Dialysis?
Home PD is an effective dialysis modality that can be suitable for almost any patient in need of dialysis. Indeed, countries with a "PD-first" dialysis policy, such as Hong Kong, have up to 80% of prevalent dialysis patients on PD. In contrast, less than 10% of dialysis patients are treated with home-based modalities in the US.(24) A sub-population that especially benefits from PD consists of those seeking independence, those who work and patients with frequent travel for work or leisure.
More than these lifestyle choices, PD should also be favored in patients with heart failure, hypotension or poor hemodynamic tolerance. With its slow and steady ultrafiltration, PD is frequently much easier to tolerate than intermittent hemodialysis and has been associated with good outcomes.(25) Similarly, elderly patients, more prone to decreased coronary reserve, might also benefit from the gentle dialysis provided by PD. The possibility of receiving dialysis treatment without having to travel from their home thrice weekly, which is necessary in CHD, is another advantage of PD in this population.(26)
Another benefit of PD that needs to be considered at the time of modality selection is that PD, compared to CHD, provides greater preservation of residual kidney function (RKF). The presence of RKF in PD has been associated with improved survival.(27) Furthermore, by allowing the use of incremental dialysis, defined as the introduction of "low-dose" dialysis in patients with significant RKF, PD can lessen the burden of dialysis therapy.
Among the relative contraindications to PD are extensive or repetitive abdominal surgeries, and patients lacking a permanent home. Other potential barriers to PD will be discussed below.
Clinical Guidelines In Peritoneal Dialysis
Guidelines on PD adequacy were published by the Canadian Society of Nephrology (CSN) in 2011.(28) Overall, measurement of RKF is essential in the evaluation of PD adequacy. Twenty-four-hour urine volume, as well as creatinine and urea clearance, should routinely be monitored in order to track changes in RKF. Monitoring also enables the treating clinician to appropriately adapt the PD prescription in patients using incremental dialysis. Use of diuretics is indicated in order to maximize urinary salt and water output. Renin-angiotensin-aldosterone (RAA) system blockers can also help preserve RKF.
Although probably not the best evaluation of adequacy in PD, a total weekly urea Kt/V >1.7, (where K equals the dialyzer clearance of urea, t equals the dialysis time and V is the patient's total body water), has been recommended by international societies. Total Kt/V should include both peritoneal and renal Kt/V.(28)
Similar to CHD, an increase in small molecule clearance has not been shown to improve outcomes in large PD-randomized controlled trials.(29)(30) However, RKF is associated with improved survival.(29) Gotch and Sargent(31) proposed Kt/V urea kinetics based on three-times-weekly hemodialysis. Because of its continuous nature, PD poorly translates into a small molecule Kt/V model. Hence, many PD facilities do not routinely measure Kt/V if not required by their dialysis organizations or payors, but rather follow clinical symptoms, RKF and laboratory values to assure adequate dialysis delivery.
The CNS guidelines for PD adequacy are available on the CSN website.
Prescription of continuous ambulatory peritoneal dialysis (CAPD) or automated peritoneal dialysis (APD) should mainly be based on patient's preference and lifestyle. Overall, most major studies have reported no significant difference in outcomes (death, technique failure) between patients treated with CAPD and APD.(32)(33)
Peritonitis is a potentially serious complication of PD. The International Society for Peritoneal Dialysis (ISPD) published recommendations centered on peritonitis prevention.(34) The monitoring of peritonitis rates is the first step of this strategy, allowing the identification of baseline rates and specific outbreaks. Since contamination at the time of connection is a major cause of peritonitis, retraining after an episode of peritonitis is also suggested.
Emptying the peritoneal cavity of dialysis fluid, along with antibiotic prophylaxis before invasive gastro-intestinal procedures, such as colonoscopy and potentially gastroscopy, are recommended in order to prevent bowel-source infections. Similar suggestions are found for dental procedures and hysteroscopy. Finally, application of topical antibiotic at the catheter exit site is recommended in order to decrease exit site infections.(34)

Table 1. Causes of Peritonitis.
- Skin or environmental contamination (intraluminal)
- Bowel-source (transvisceral migration)
- Catheter related infection (periluminal)
- Bacteremia (hematogenous)
- Gynecologic source

ISPD guidelines for prevention and treatment of peritoneal dialysis-related infections are available on the ISPD website.
How to Assess and Follow Patients Undergoing Peritoneal Dialysis
PD allows patients to receive their dialysis treatment in a home environment. Nonetheless, regular follow up, perhaps every one to three months, is necessary to monitor dialysis quality. In the United States, the Centers for Medicare and Medicaid Services (CMS) mandates monthly evaluation. Clinical assessment should first evaluate the presence of any uremic symptoms. Weight loss, decrease in appetite or energy level can all be signs of reduced clearance, either secondary to non-adherence, changes in RKF or, more rarely, a change in the transport characteristics of the peritoneal membrane.
Reduction in clearance may also be indicated by increased serum creatinine, urea or phosphate levels, which should be routinely monitored, especially in patients with incremental PD. Since phosphate clearance is 'time-dependent,' acting as a larger molecule despite its small molecular size, patients undergoing nocturnal intermittent peritoneal dialysis (NIPD) and are thus without dialysis for the majority of the day, can be specifically prone to an increase in phosphate level, especially when their RKF declines.(35)(36)
With the exception of providing a long dwell time (i.e., the time of contact between the dialysate and the peritoneal membrane) for appropriate phosphate clearance, mineral bone metabolism management in PD is similar to that in hemodialysis and based on calcium, phosphate and parathyroid hormone levels.
Anemia management with iron or intravenous supplements and erythropoietin-stimulating agents (ESA) should also follow general international anemia guidelines, targeting a hemoglobin around 100 to 115 g/L.(37) It is more complicated to administer iron intravenously in the outpatient setting, and patients may have to make a special visit to the clinic or hospital to receive parenteral iron. It should never be given intraperitoneally.
Volume status also needs to be closely monitored in PD. Elevated home or clinic blood pressures, presence of lower leg edema and dyspnea can all be signs of volume overload. Here again, a careful evaluation should be performed to identify the underlying cause. Specific attention should be directed toward non-adherence to PD prescription or dietary salt restriction, reduction in RKF or a reduction in the ultrafiltration (UF) capacity of the peritoneal membrane. UF can usually be improved by increasing dialysate dextrose concentration, reducing dwell time or the use of icodextrin. Maximization of residual urine output through diuretics should never be forgotten.
Finally, exit sites should be evaluated on a regular basis by the patient at home and by the nursing or medical team at time of clinic visits. Discoloration, redness and discharge around the exit site should raise the suspicion of exit site infection.
Management of Comorbidities Associated With Peritoneal Dialysis
Patients undergoing PD are usually subject to similar complications as other ESRD patients, namely mineral bone disease, anemia and cardiovascular disease. Assessment of nutritional status might, however, be particularly challenging in patients treated with PD. Albumin levels are commonly lower in PD than HD, perhaps because of its loss through the peritoneal membrane Consequently, the relationship between serum albumin and outcome is not as close in PD as it is in HD. The assessment of malnutrition generally requires the combination of different parameters, including anthropometric and physical examination, laboratory evaluation, clinical tools such as the 'Subjective Global Assessment' and a clinical evaluation (uremic symptoms, loss of RKF, chronic inflammation).(38)
Addressing Potential Barriers to Peritoneal Dialysis
A barrier frequently identified as a limitation to PD expansion is the need for the patient to be totally independent. Although PD is a home-based modality allowing a great level of autonomy, complete independence is not crucial to its success. Caregiver-assisted PD, where most of the dialysis is undertaken by the caregiver, is associated with good clinical outcomes, with the caveat that these dependent patients often have a higher burden of comorbidity.(39) The burden of care on these caregivers can frequently be decreased by modeling the PD prescription to the caregiver lifestyle, for instance by changing from CAPD to APD to suit a working schedule. Overall, caregivers do not experience a significant decrease in their quality of life while performing PD for a dependent patient.(40)
PD can also be performed by home-care nurses, defined as nurse-assisted PD, allowing elderly or frail patients without available caregivers to benefit from PD in their own home environment. Similarly, nursing homes with PD-trained staff allow elderly patients to continue or initiate PD once they need more sustained health care. Although outcomes such as survival, peritonitis and hospitalization are sometimes (but not always) poorer in patients with assisted-PD, the overall benefits of a home technique, such as the avoidance a thrice-weekly transportation and the more gentle fluid removal, need to be considered for these frail patients.(41)
Another important barrier to PD is the lack of dialysis planning or the inability to start PD in a patient acutely presenting with ESRD. Although timely dialysis education through dedicated pre-dialysis teams remains the optimal transition to home-based dialysis, patients presenting with an urgent need of dialysis initiation can still be directed to PD. The implementation of an in-hospital chronic kidney disease (CKD) education program specifically designed for these acute starts can allow a significant portion of patients to select PD.(42) Furthermore, timely insertion of PD catheter, either by interventional radiology or bed-side technique, can also allow a greater number of patients to start PD more acutely.(43)
Summary
Home-based dialysis modalities, including PD, are performed by a minority of dialysis patients in the US. Knowing the numerous benefits of PD, notably independence, preservation of RKF and slow and steady UF, this modality should be offered to a greater number of CKD patients. Overall, the follow-up of PD is based on the assessment of clinical symptoms, routine blood work and physical signs of fluid balance. Finally, in order to increase its availability, PD assisted by caregiver or home-care nurses should be offered by PD units. Implementation of acute CKD education programs and timely insertion of the PD catheter should also be targeted as measures to overcome barriers to PD expansion.