The Weathering Hypothesis of Aging: Part II
Course Authors
Bruce S. McEwen, Ph.D.
Dr. McEwen reports no commercial conflict of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Describe how the HPA axis is controlled by a number of extra-hypothalamic brain structures which have inputs to the hypothalamus
Outline how individual differences in HPA activity arise in animal models from early-life experiences
Discuss individual differences in "weathering", i.e., rate of aging of the human brain and show how they may parallel animal models.
Introduction
"Weathering" is another way of describing allostatic load, which refers to the wear and tear on the body and brain from either too much stress or from the inefficient management of the hormonal stress response. Previous Cyberounds® have developed the notion of allostatic load as it applies to the metabolic and cardiovascular systems, to the immune system and to the brain, and many of the examples of allostatic load have been seen in elderly individuals.
Recent research on animal models has shown that the hypothalamo-pituitary-adrenal (HPA) axis (see Figure 1) is central to a life-long pattern of hormonal responses that may cause the brain to age more or less slowly. The hippocampus appears to play a central role in its own "weathering" because it is both the target of stress hormones and, also, a controller of HPA activity. The hippocampus, along with other brain structures, controls output of CRF from the hypothalamus which controls ACTH release from the anterior pituitary gland. It is the negative feedback of adrenal steroids on the brain and pituitary that ultimately controls HPA activity and contains the output of stress hormones.
However, as Figure 1 also shows, the role of the hippocampus in HPA regulation is more complex than originally believed, considering both its neuroanatomical connections to the hypothalamus and the nature of negative feedback regulation of the HPA axis.
In this Cyberounds®, we shall examine the role of the hippocampus in HPA axis regulation. We will then consider how the development of the hippocampus plays a key role in establishing a life-long pattern of reactivity of the HPA axis and, thus, helps set the rate of "weathering" of the brain and body.
The Hippocampus and HPA regulation
The hippocampus is involved in the regulation of HPA activity,(1) although the nature of this regulation is more complex than originally suspected. In general, the hippocampus has a consistent inhibitory effect on HPA axis reactivity(1),(2). Glucocorticoid implants into the hippocampus produce an inhibition HPA activity,(1) whereas the amygdala has a generally facilitative role.(3),(4),(5) Hippocampal lesions produced elevated cortisol secretion under a variety of stressful and non-stressful conditions, although the results reported in the literature are not entirely consistent with a feedback role of the hippocampus in HPA regulation (see 1). However, lesion and steroid implant experiments also reveal an equally important role for the medial prefrontal cortex in HPA regulation.(6) As shown in Figure 1, the neuroanatomical links from the hippocampus and medial prefrontal cortex to the hypothalamus are postulated to be via the bed nucleus of the stria terminalis and preoptic area, with an output from these structures to the paraventricular nucleus via inhibitory gamma amino butyric acid (GABAergic) projections.(7)
Figure 1.
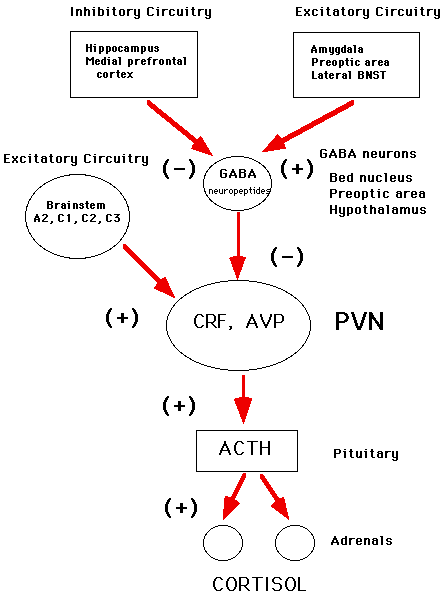
Based upon J.P. Herman, W.E. Cullinan, Trends in Neuroscience 20: 78-84, 1996.
A considerable amount of data has accumulated showing that elevated HPA activity is correlated with reduced levels of Type I or Type II receptors in the hippocampus
It is unclear whether correlations of hippocampal Type I and Type II receptor levels with HPA activity indicate a feedback action of adrenal steroids on the hippocampus, or a priming role for glucocorticoids to make the HPA axis optimally reactive to turning on and turning off a stress response, as suggested by recent studies by Dallman and colleagues.(12) In this study, it was shown that a constant level of glucocorticoids from pellets implanted into adrenalectomized rats normalized the hypersecretion of ACTH resulting from adrenalectomy, but the same result was obtained by giving glucocorticoids to adrenalectomized (ADX) rats in their drinking water, which the rats drink mainly in the dark phase of their diurnal cycle when they are awake and exploring their environment.(12) The big difference between the two modes of glucocorticoid replacment was seen in the dynamics of how ACTH was secreted in response to stress: in rats consuming corticosteroids in the drinking water, ACTH release was increased by stress and turned off efficiently after the termination of stress, whereas in the rats with corticosteroid pellets, ACTH was secreted in stress but was turned off very slowly after the end of the stress.
Thus, adrenal steroid secretion by stress is not necessary for terminating ACTH secretion (because ADX rats do not produce glucocorticoids when stressed!) and the diurnal pattern of adrenal steroid exposure may be a key to the dynamics of the ACTH response to stress. It is this last aspect of HPA regulation that is the most intriguing: namely, that the role of the hippocampus may be as an adrenal steroid-primed modulator of neural activity that is involved in regulating hypothalamic output of CRF and vasopressin.
How Does HPA Activity Change with Age?
Many but not all studies show increased levels of glucocorticoids in aging rats and humans.(13),(14),(15),(16),(17)The reasons for these differences are likely to relate to individual differences in brain aging and in the differences in the distribution of aging, impaired individuals in populations of animals and human subjects.(14) Particularly useful have been studies of basal cortisol levels and cognitive deficits in human aging.(18),(19) Aged subjects followed over a four year period, who showed a significant increase in cortisol levels over the four years and had high basal cortisol levels in year four, showed deficits on tasks measuring explicit memory as well as selective attention, compared to subjects with either decreasing cortisol levels over four years or subjects with increasing basal cortisol but moderate current cortisol levels.(18) They also showed a hippocampus that was 14% smaller than age-matched controls who did not show progressive cortisol increases and were not cognitively impaired.(19)
Elevated and disregulated HPA activity is also seen in depressive illness(20),(21) but the reasons for this elevation are not entirely clear. Disregulation of the HPA axis associated with major depression is revealed by the dexamethasone suppression test (DST).(22),(23) Although the DST is most likely working at the pituitary level,(24) the underlying disregulation is undoubtedly of CNS origin and reflects increased drive upon the CRH and AVP systems of the hypothalamus(25) and constitutes a form of endogenously driven stress. A lessening of the adrenal steroid feedback effects on the hippocampus might be a contributing factor to elevated HPA activity in depression, and recent studies of Barden and coworkers with a transgenic mouse strain(26),(27) have suggested that decreased forebrain Type II receptor expression might be a contributing factor to depression and a potential target of antidepressant therapy.(28) It is not clear, however, how much hippocampal Type II receptor expression, as opposed to expression in other brain regions, is a key factor.
Hippocampal atrophy and elevated glucocorticoid levels in those individuals(29),(30),(31) raise the "chicken-and-egg" question, namely, whether hippocampal atrophy is both a cause and a result of the elevated glucocorticoids and whether the cognitive impairment accompanying these conditions is due to the hippocampal atrophy or to elevated glucocorticoids affecting neuronal excitability, or both. The acute effects of glucocorticoids on memory are recognized and have been reviewed recently.(32) In general, an acute elevation of glucocorticoids by injection causes acute cognitive impairment of declarative memory in human subjects.(33) Nevertheless, in spite of one positive report,(33) it is not as certain whether acute stress-induced elevations of corticosteroids can do the same.(34) This may be a question of the magnitude of the stress-induced cortisol rise, since a bolus injection of cortisol not only impairs declarative memory but also suppresses temporal lobe uptake of glucose.(35)
The Problems and Opportunities of Studying Individual Differences
We now return to the question of individual differences in brain and body aging and discuss the possible determinants of these individual differences. There are two major factors: genetic constitution and environmental influences, and we know that gene expression is regulated by environmental factors and that hormones play a major role in the regulation of gene expression. Therefore, the discussion of the determinants of individual differences can be framed, at least in part, as a question of how experience influences brain development and adult function.
The vulnerability of many systems of the body to stress is influenced by experiences early in life. In animal models, unpredictable prenatal stress causes increased emotionality and increased reactivity of the HPA axis and autonomic nervous system and these effects last throughout the lifespan. Postnatal handling in rats, a mild stress involving brief, daily separation from the mother, counteracts the effects of prenatal stress and results in reduced emotionality and reduced reactivity of the HPA axis and autonomic nervous system.(36),(37),(38)
The vulnerability of the hippocampus to age-related loss of function parallels these effects - prenatal stress increasing and postnatal handling decreasing the rate of brain aging.(39),(40) Concurrently, age-related decline of gonadal function reduces the beneficial and protective actions of these hormones on brain function. At the same time, age-related increases in adrenal steroid activity promotes age-related changes in brain cells that can culminate in neuronal damage or cell death. Life-long patterns of adrenocortical function, determined by early experience, contribute to rates of brain aging, at least in experimental animals.
Unpredictable or uncontrollable stressful experiences of a pregnant rat increase emotionality and stress hormone reactivity in offspring that last for the lifetime of the individual, whereas the gentle and repeated stimulation of newborn rat pups, known as postnatal handling, produce reductions in emotionality and stress hormone reactivity that also last a lifetime.(41),(42),(43),(44) These effects appear to involve mediation by both the mother's behavior and by adrenal and thyroid hormone actions.
More is known about the mechanism of neonatal handling. Handling involves separating the pups from the mother for 10 minutes per day for the first two weeks of neonatal life and the licking of the pup by the mother appears to be an important determinant of the postnatal handling effect.(45) At the same time, increasing corticosterone levels in the mother's milk mimics some of the effects of neonatal handling.(46) Thyroid hormone elevations have been suggested as a possible mediator of the neonatal handling effect, particularly regarding the elevated expression of glucocorticoid receptors in the hippocampus.(47)
Studies, in which both prenatal stress and postnatal handling were compared, indicate that these two procedures have opposite effects on food intake, body weight and anxiety, as well as HPA activity.(48) However, the two processes interact, in that prenatal stress effects on HPA activity and emotionality are reversed by early postnatal 'adoption' or cross-fostering of pups to new mothers,(49),(50) which is most likely a form of postnatal handling involving intense licking of the pup by the mother.(45) Prenatal stress during the last week of gestation in rats increases reactivity of the HPA axis and reduces expression of the Type I adrenal steroid receptor in the hippocampus, which helps to contain basal levels of HPA activity.(51) Prenatal stress also increases anxiety in an open field test and decreases basal food intake and body weight.
It is important to note that some of these prenatal stress effects may involve mediation by adrenal steroids.(52) Taken together with the fact that postnatal handling effects may also be mimicked by adrenal steroids,(46) the specific effects of adrenal steroids on the neural development of emotionality and HPA reactivity may change qualitatively as the nervous system matures.
For prenatal stress and postnatal handling, once the emotionality and the reactivity of the adrenocortical system are established by events early in life, it is the subsequent actions of the hypothalamo-pituitary-adrenal (HPA) axis in adult life that play a major role in determining the rate of brain and body aging. Increased HPA activity is associated with increased brain aging, whereas the opposite is true of animals with reduced HPA reactivity to novel situations. Rats with increased HPA reactivity show early decline of cognitive functions associated with the hippocampus(40) as well as increased propensity to self-administer drugs such as amphetamine and cocaine.(53),(54) In contrast, rats with a lower HPA reactivity, as a result of neonatal handling, have a slower rate of cognitive aging and a reduced loss of hippocampal function.(55)
Is There a Human Counterpart to the Story of Individual Differences in Rat HPA Activity and Hippocampal Aging?
We simply cannot say at this moment, but individual differences in human brain aging that are correlated with cortisol levels have been recognized in otherwise healthy individuals who are followed over a number of years.(56) In the most extensive investigation, healthy elderly subjects were followed over a four-year period. Those who showed a significant and progressive increase in cortisol levels during yearly exams over the four years and had high basal cortisol levels in year four showed deficits on tasks measuring explicit memory as well as selective attention, compared to subjects with either decreasing cortisol levels over four years or subjects with increasing basal cortisol but moderate current cortisol levels.(18) They also showed a hippocampus that was 14% smaller than age-matched controls who did not show progressive cortisol increases and were not cognitively impaired.(19) In the other study of successful aging, increases in overnight urinary cortisol secretion from 1988 to 1991 predicted declines in cognitive function in women, while the effect was not significant in men.(56) This finding is being extended in a new data collection (Dr. Teresa Seeman, UCLA, personal communication).
Conclusions
Developmental influences on HPA activity and rates of hippocampal aging provide a compelling model for life-long patterns of human aging but we do not have longitudinal studies for human beings that would confirm that these predictions are correct. What we do have are the indications that individual differences in HPA activity do exist in aging humans, more or less as they occur in aging rats, and that elevated HPA activity does predict aspects of "weathering," including cognitive decline. We also have examples showing how these individual differences in life-time experiences and in patterns of HPA activity in brain aging affect the body and this brings us back to the concept of allostatic load developed in previous Cyberounds®.
"Weathering" of the brain and body at different rates in different people is likely to be the product, at least in part, of individual differences in allostatic load, resulting either from the amounts of stress in one's life or from the inefficiency with which the hormonal stress response and the diurnal rhythm of glucocorticoid secretion are regulated. Thus, the greater "weathering"` of individuals experiencing economic hardship in their lives may be attributable to greater amounts of chronic stress,(57) whereas the increase rates of bone demineralization in depressed women is explainable by the increased levels of cortisol throughout the diurnal cycle that are part of the hyperactivity of the HPA axis in depression.(58) Moreover, increased glucose levels and insulin resistance in people undergoing sleep deprivation are traceable to elevated evening levels of cortisol, another example of an inefficient diurnal rhythm that can exacerbate pathophysiology;(59) and this condition is likely to result in increased abdominal obesity, muscular weakening and possibly also cognitive impairment if it is continued over long periods of time.(60)
Now that we have discussed the bad news, it is time to re-examine the positive side of the story, including what can be done to moderate the age-related decline in cognitive function. We begin in the next Cyberounds® with a discussion of the dynamic plasticity of the hippocampus.
Acknowledgments
Research in the author's laboratory on some of the topics discussed in this article is supported by NIH Grants NS07080 and MH41256 and by the Health Foundation (New York), Servier (France) and UCB (Belgium).