Course Authors
Susan C. Stewart, M.D.
Dr. Stewart reports no commercial conflict of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the current information on location and expression of the breast cancer susceptibility genes
Recognize the difference between somatic and germline mutations and their relative frequency in breast cancer patients
Access the Cooperative Family Registry for Breast Cancer Studies.
Everyone of us has had a patient, friend, or relative concerned about their family history of breast cancer. With the rapid evolution in this field in the last few years, much new information has been developed. My goal for this Cyberounds® is to bring you up-to-date on this information, to set it in its proper perspective and to give you resources to refer to for your practice and your patients.
For this conference I have asked Dr. Anne Moore to review the information. She is Professor of Clinical Medicine of Cornell University Medical College, Chair of the Hematology Section of the American Board of Internal Medicine, President of the New York Metropolitan Breast Cancer Group and a highly respected expert on breast cancer. She is the author of The Patient's Guide to Breast Cancer Treatment which is now available on the Internet.
Incidence and Risk of Breast Cancer
Breast cancer is the commonest cancer in women. In 1997, 185,000 women in the United States are expected to be diagnosed with the disease, making it the third most deadly cancer behind lung and colorectal cancer. But the good news is that, with greater use of mammography and diagnostic techniques that permit diagnosis at earlier stages, the cure rate is improving.
In the last 10 years, a great deal of emphasis has been given to the estimate that every woman faces a one in eight chance of developing breast cancer during her lifetime. This is an alarming figure and needs to be placed in its proper perspective. First of all, this is a life-time risk, with potential lifetime of 110 years. Second, it is cumulative risk, the one in eight figure being reached at the end of a long life. The risk at age 50 is 1 in 50. (see Table 1)
Table 1. A Woman's Risk of Developing Breast Cancer by Age.
| By age 25 | one in 19,608 |
| By age 30 | one in 2,525 |
| By age 35 | one in 622 |
| By age 40 | one in 217 |
| By age 45 | one in 93 |
| By age 50 | one in 50 |
| By age 55 | one in 33 |
| By age 60 | one in 24 |
| By age 65 | one in 17 |
| By age 70 | one in 14 |
| By age 75 | one in 11 |
| By age 80 | one in 10 |
| By age 85 | one in 9 |
| Ever | one in 8 |
Source: NCI.
Surveillance Program
Separating Somatic From Germ-line Mutations
Most cases of breast cancer, about 90%, are thought to be the result of sporadic, somatic mutations in the breast tissue itself. The other 10% are associated with germline, or inherited mutations, that can greatly increase the chance of breast cancer in an individual. It is important to keep these concepts separate in your own mind, even though they may converge in a single patient. There is always a background risk for somatic mutation and breast cancer in any woman, which will be increased by the commonly cited endogenous and environmental risk factors. The risk conferred by a germline mutation, in the BRCA1 or the BRCA2 genes, or mutations of other genes yet to be identified, must be added to that background risk. A practical consideration from this knowledge is that a woman with a BRCA1 gene mutation in her family, who tests negative for that mutation, is not without risk for breast cancer. She still faces the one in eight lifetime risk for a somatic mutation and should continue surveillance accordingly.
Both BRCA1 and BRCA2 mutations confer increased risk for breast and ovarian cancer as well as for other cancers. They are tumor suppressor genes, and when mutations alter or inactivate this function, cancer is more likely to develop. These genes were discovered through studies of families with multiple cases of breast cancer in different generations. First, linkage studies, then actual gene sequencing pinpointed the location of the genes and the exact mutations. BRCA1 was sequenced in 1994 and BRCA2 was sequenced in 1995. Table 2 summarizes key information about the effect of mutations in these two genes.
Table 2. Effects of Mutations in BRCA1 and BRCA2.(1),(2),(3)
| BRCA1 | BRCA2 | Bkgrd Risk | |
| Chromosome | 17 | 13 | |
| Year Discovered | 1990 | 1994 | |
| Year Isolated | 1994 | 1995 | |
| Mutations | 100 | 100 | |
| Breast cancer | 56-85% | 56-85% | 10-12% |
| Ovarian cancer | 26-85% | < 10% | 1% |
| Male Breast cancer | no | yes | |
| Other cancers | prostate | colon |
The mode of inheritance of these mutations is autosomal dominant. For a long time, many of us thought that the breast cancer susceptibility was inherited through the maternal relatives. This is not the case with the BRCA genes. Cases of breast and ovarian cancer may seem to skip a generation when being carried by a male, but phenotypic expression of the mutation will occur in females in the next generation.(4)
Figure 1. Four-Generation Pedigree Showing Autosomal Dominant Inheritance of a Breast Cancer Gene.
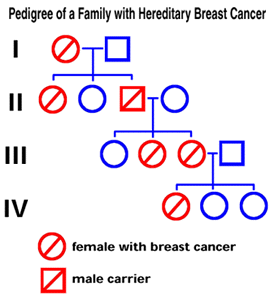
For simplicity, 100% penetrance is assumed. Breast cancer occurs in females in four generations. Note that the male carrier in Generation II has two daughters and a granddaughter with breast cancer.
Remember that the gene is one complex area on a single chromosome. Within that gene are multiple areas where alterations (mutations) in the base-pair sequences have been found to occur, over 200 in the BRCA1 and BRCA2 genes combined. A specific mutation can occur in a single family. In studies that determine the spectrum of mutations, some are very common. One such is the 185delAG (deletion of an adenine and guanine in codon 185), which has been found in 21% of women of Ashkenazi Jewish descent who have developed breast cancer before the age of 35.(5) Ninety percent of Jews in the United States are Ashkenazi and over 2% of this group carry either BRCA1 or BRCA2 mutations.(1),(7) These families have been studied intensively because of this high incidence.
What Do All These Many Mutations Mean?
Not all mutations result in phenotypic expression. Some mutations are in silent areas of the gene and are never expressed. Some mutations have variable penetrance: phenotypic expression occurs in one family member but not in another with the same mutation. A recent study demonstrated a 56% risk of breast cancer in BRCA1 and BRCA2 carriers.(1) The original families that were studied had a very high incidence of cancer cases and may represent a mutation with extremely high penetrance, which may not be the case for carriers of mutations in other families.
Another question that needs investigation is how do these mutations, this inherited susceptibility, interact with other identified risk factors for breast cancer? We know that exposure to endogenous and exogenous estrogen is a primary risk factor. How much of a contribution does an environmental factor make to expression of the mutated gene? Would controlling this exposure protect a woman with a mutation?
Before the BRCA genes were located and mutations identified, observational studies of cancer families permitted calculation of risk for breast and ovarian cancer in women of varying ages, depending on family characteristics -- the degree of relationship and the age at which the relative developed breast cancer.(6)
Now, in the case of the known BRCA gene mutations, it is possible to pinpoint risk in many individuals. It is important to keep in mind, when assessing the degree of a patient's risk for breast cancer in a family in which a particular mutation is identified, that even a woman without that mutation would still have the background risk for breast cancer of 10%.
Weighing The Cost of Testing Against the Possible Benefit of Diagnosis
There are already 200 mutations identified on BRCA1 and BRCA2. When genetic testing is first done on material from a patient in a high risk family, the entire BRCA1 and BRCA2 genes may have to be examined to determine which one of the many mutations is present. This could cost $2500 or more. If a specific mutation is identified, then other family members can be tested for a considerably lower cost of probably less than $500, because the precise area to be analyzed is known. Such testing is not typically covered by health insurance policies.
The Importance of Counseling
Far more important than cost is consideration of what the information from testing might mean and what effect it might have on a given patient and on her family. Genetic testing is currently considered a research tool, which requires pretest and post-test counseling about what the potential results could be and what they mean, as well as support and follow up for dealing with this knowledge.(3) Expert counseling is crucial whenever genetic testing is undertaken.
When to Consider Testing
What are the family characteristics that would raise suspicion that a patient might have a mutation in a susceptible gene and so be a candidate for genetic testing? Here is a list of preliminary questions:
- Breast or ovarian cancer at an early age in patient or relative
- A relative with breast and ovarian cancer
- A male relative with breast cancer
- Two or more relatives with breast or ovarian cancer
The closer the relationship of these relatives to the patient, the higher the probability that a mutation is present, especially first degree relatives (parent, sibling, or child).
However, physicians also need to inject a note of caution into genetic testing. A woman tested against a panel of known mutations on a particular gene may have none of them and be judged negative. She might still have a yet undiscovered mutation on that gene or on another gene altogether.
We are currently in a situation where technology is progressing faster than individuals, society, ethics and business can deal with it. The privacy issues are much the same as those for HIV testing. In addition, as discussed in detail in a previous Cyberounds® Health Law and Bioethics Conference, information about genetic susceptibility could lead to discrimination in health insurance, life insurance and employment. Even going to have the test may lead to adverse consequences.
The Cooperative Family Registry for Breast Cancer Studies
For these reasons and because not all cases of what looks like hereditary breast cancer are explained by the presence of the known mutations, physicians may want to recommend that their patients at risk consider joining The Cooperative Family Registry for Breast Cancer Studies. The Registry is a program funded by the National Cancer Institute to create a databank of specimens from individuals with a family history (including personal history) of breast and/or ovarian cancer. These specimens, along with detailed health and life style information about the subject and information about affected family members, will be available to researchers. Each participant is asked to complete a yearly follow-up questionnaire.
This program is not about testing individuals. All personal identifying information is separated from the databank information and specimens. Participants in the program will learn about research progress (which may be relevant to them) from bi-annual newsletter reports. Educational programs will also be offered to Registry participants. The most up-to-date recommendations on surveillance will be available to participants.
The Registry offers patients a place where they can gather information, gain a sense of control (at a time when that can be extremely valuable) and make a contribution to a field in which knowledge is rapidly progressing and our perspective changing all the time. For example, there may be additional mutations of the currently identified genes and there may be other, yet unidentified susceptibility genes. Similarly, endogenous (i.e., estrogen exposure) and environmental (radiation) risk factors may influence the expression of various types of gene mutations. Researchers using Registry specimens will be able to analyze the clinical information and, hopefully, clarify these interactions.
This Registry is available at centers all over the United States as well as at international locations -- currently, Australia and Canada. There are six centers in the New York City area, designated collectively, as the Metropolitan New York Registry of Breast Cancer Families. Each center can provide referral for genetic counseling and testing or for expert breast and ovarian cancer care. To learn more about locations and how to reach the Project Coordinators, consult the NCI Webpage. Participating medical centers are currently putting Registry information on their own web pages.
Medical professionals should encourage their patients to join the Registry or similar programs in their country. It is a very positive way for women with a family history of breast cancer who do not want to be tested or who test negative to make a contribution to scientific advancement in breast cancer genetics an also keep up-to-date on developments in the field, which may be personally beneficial.