Course Authors
M. Louay Omran, M.D.
Dr. Omran is Assistant Professor in the Division of Geriatrics at Saint Louis University School of Medicine. Dr. Omran reports no commercial conflict of interest.
This activity is made possible by an unrestricted educational grant from the Novartis Foundation for Gerontology.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the basic physiology of swallowing and its associated disorders
Discuss an approach to the diagnosis of dysphagia
Recognize the available management options for oropharyngeal and esophageal dysphagia.
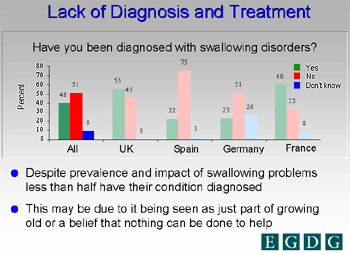
Dysphagia is the term developed from Latin roots used to describe the presence of difficulty in swallowing (dys =difficult, phagia=swallow). It is a common problem in the United State with estimates of about 10 million Americans being evaluated each year for swallowing difficulties. Despite its prevalence in older persons, dysphagia is likely to be undertreated, as shown in a recent European study.
Figure 1. Lack of Diagnosis and Treatment. Courtesy of Dr. O. Ekberg.
Physiology of Swallowing
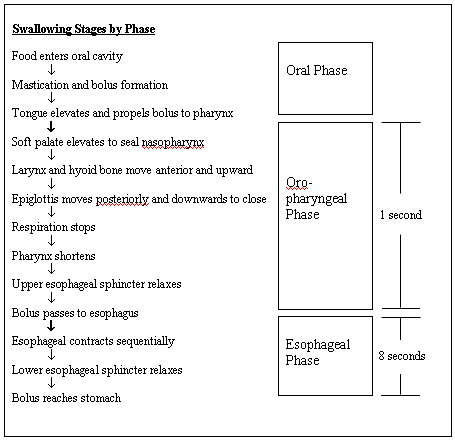
The swallowing process consists of a programmed dynamic sequence of muscular contraction and relaxation. It involves more than 40 pairs of muscles and can be divided into three phases based on the location: the oral, oro-pharyngeal, and esophageal phase.(1) The term "dysphagia" is used to refer to problems in any of the swallowing phases and can be prefixed by the name of the specific phase.
The oral phase is a voluntary phase that includes preparing the food bolus, then launching it into the back of the oral cavity towards the pharynx.
The second phase starts, thereafter, with several sequential activities including elevation of the soft palate to seal the oropharynx, anterior displacement of the arytenoid cartilage and the larynx, posterior movement of the epiglottis and temporary respiratory cessation to protect the airways. The bolus is then pushed downwards towards the esophagus as a result of pharyngeal peristaltic contractions behind the bolus. The cricopharyngeal muscle, also known as the upper esophageal sphincter (UES), then relaxes and the food is propelled into the esophagus. This oropharyngeal phase is reflexogenic, controlled by the swallowing center in the medulla and mediated through several cranial nerves (V, IX, X, XI, and XII).(2)
The esophageal phase starts with the bolus reaching the upper third of the esophagus which, similar to the oropharynx, is composed of striated muscles innervated by the IXth and Xth cranial nerves. The bolus moves downwards following continuous peristaltic contractions sweeping through the upper third of the esophagus into the lower two thirds which are composed of smooth muscles innervated by the dorsal motor nucleus of the vagus nerve and by the neurons in the myenteric plexus. Shortly before the arrival of the bolus, the lower esophageal sphincter (LES) relaxes, allowing the bolus to fall into the stomach. The oropharyngeal phase lasts about one second while the esophageal phase lasts about eight seconds.(3)
Figure 2. Swallowing Stages by Phase.
A disruption of any stage of this complicated process can impede the progress of the bolus and result in dysphasia. The disruption could be caused by either neuromuscular failure affecting the peristaltic aspect of swallowing or mechanical impediments affecting the clear passage of the bolus.
Aging brings along physiologic changes in the swallowing process that can possibly cause difficulty in swallowing. Most studies examining swallowing in healthy older persons reflect increased rigidity of the upper esophageal sphincter (UES). The rigidity can result in an increased resistance to food passage that mandates an increase in the velocity of pharyngeal contraction. Aging can also result in a decrease in the number of myenteric ganglionic cells that coordinate esophageal peristalsis. This, however, does not cause any impairment in the movement of food down the esophagus. In the past, abnormal esophageal spasms in older persons were attributed to aging and termed "presbyesophagus". Currently, this abnormal peristalsis is thought to be due to disease processes.
Causes of Dysphagia
Dysphagia can be divided into two categories based on the location of the problem. The term "oropharyngeal dysphagia" is used if the problem arises prior to the bolus reaching the upper esophagus. "Esophageal dysphagia" is used if the problem arises afterwards.
The most common causes of dysphagia in young people include inflammatory muscle diseases, webs and rings. Webs are mostly located in the upper third of the esophagus and are either congenital or inflammatory. In some occasions, webs are not symptomatic, though a concentric web can cause intermittent dysphagia to solids. The presence of a symptomatic hypopharyngeal web, in addition to iron deficiency anemia, is termed Plummer-Vinson syndrome. Schatzki's rings are a common cause of dysphagia. They may be related to acid reflux and are usually located in the lower third of the esophagus above the LES.
In older persons, oropharyngeal dysphagia is usually caused by central nervous system lesions including strokes, Parkinson's and dementias. Esophageal dysphagia commonly results from reflux esophagitis, motility disorders and tumors. Reflux esophagitis is an inflammatory process provoked by recurrent mucosal injury in the lower esophagus. Acid reflux is thought to be due to intermittent relaxation of the LES and is worsened by the presence of a hiatus hernia.
Chronic reflux esophagitis can lead to a stricture and a resulting dysphagia. It can also cause replacement of the normal esophageal squamous epithelium by columnar epithelium. This condition is known as "Barrett esophagus" and can be further complicated by peptic stricture or adenocarcinoma in up to 5% of cases. Most cases of esophageal cancer are squamous cell carcinomas. Approximately 15% of cases occur in the upper third of the esophagus, 50% in the middle third and 35% in the lower third.
The risk of esophageal cancer increases with age. Other risk factors include alcohol and tobacco abuse, injury from physical agents (radiation therapy, ingestion of chemicals) and pre-malignant conditions, such as achalasia and Plummer-Vinson syndrome.
Idiopathic achalasia is a motility disorder that is associated with dysphagia. Pathophysiologically, there is a significant reduction in the myenteric neurons leading to the loss of esophageal peristalsis and the inability of the LES to relax. Idiopathic achalasia should be differentiated from secondary achalasia, possibly caused by conditions such as Chagas disease or cancers infiltrating the esophagus.
Diffuse esophageal spasms (DES) are disorders of smooth muscle contraction affecting the lower two thirds of the esophagus. The spontaneous, large amplitude, repetitive contraction can cause chest pain and dysphagia to liquids more than solids. Etiology is thought to be secondary to neurologic degeneration or to internal esophageal irritation.
A list of the most common causes of dysphagia is provided in Table 1.
Table 1. Common Causes of Dysphagia.
| Oropharyngeal | Esophageal |
Obstructive/Mechanical
| Intrinsic Obstructive Lesion
|
Clinical Presentation
History
To make a preliminary diagnosis of dysphagia and choose the appropriate diagnostic test, it is essential to obtain an accurate history. History should cover the location of discomfort, time of onset, accompanying symptoms, type of food and progression over time.(4),(5)
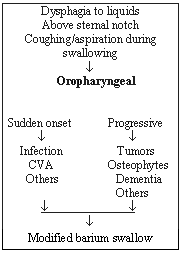
Oropharyngeal dysphagia typically causes difficulty in swallowing liquids. The patient complains of food "sticking" in the throat, usually above the sternal notch. It happens immediately after swallowing and may be accompanied by coughing, choking or nasal regurgitation. When the onset of these symptoms is acute, it may suggest a stroke; if it is progressive, it may suggest head or neck tumor.
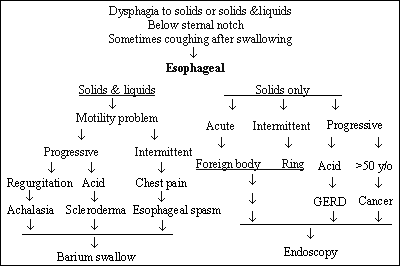
Esophageal dysphagia, typically, is perceived with discomfort in the substernal ( lower third location is typical) region. However, pain may be referred to the suprasternal area and, as a result, confused with oropharyngeal dysphagia.(6) This is often considered to occur about 30% of the time when the lesion is actually distal. Discomfort usually presents shortly after swallowing and, depending on the etiology, may be present with solids only, if it is obstructive, or both solids and liquids, if it is motility related.
The presence of angina-like chest pain usually suggests esophageal spasm or gastro-esophageal reflux. Painful sensation, sensed for the few seconds required for the food to pass down the esophagus, often referred to as odynophagia, usually reflects esophagitis, secondary to infections, chemical burn or lodging of pills. Regurgitation of food shortly after eating and or halitosis may suggest Zenker's diverticulum (usually appearing in the posterior wall of the hypopharynx). Presence of weight loss can suggest malignancy.
When solid food dysphagia is intermittent, benign rings (especially lower esophageal rings) are usually the cause, while malignancy is suspected when dysphagia is progressive. Intermittent solid and liquid dysphagia usually reflects esophageal spasm. A progressive dysphagia reflects achalasia (failure of the muscular ring to relax) in the absence of acid reflux or scleroderma if reflux is present.(7)
The algorithm in Figure 3 can be used to classify patients and chose the appropriate diagnostic test.
Figure 3. Patient Classification and Diagnostic Algorithm.
Dysphagia should not be confused with the purely sensory symptom, "globus hystericus," in which swallowing is unimpaired. Globus is a condition characterized by the sensation of fullness in the throat usually between meals and may be relieved by eating. Of unknown etiology, it is possibly psychological.(8)
A summary of the most frequently encountered complaints in older persons suffering from dysphagia (as shown in the unpublished PanEuropean study) is shown in Figure 4.
Figure 4. Prevalence of Symptoms.
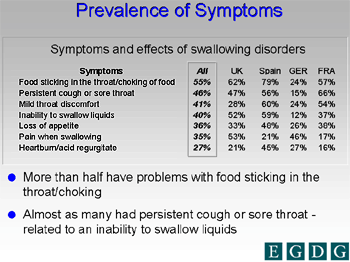
Courtesy of Dr. O. Ekberg.
Physical Examination
The physical examination should not only be focused on the local organs involved in the swallowing process but also be expanded to include signs of systemic disease and possible sequelae of dysphagia (e.g., aspiration pneumonia and malnutrition).
Head and neck examination may reveal signs of previous surgery, radiation or tracheostomy. An otolaryngologist should perform a thorough oro-pharyngeal examination to evaluate dentition, the tongue, oropharynx, hypopharynx and vocal cords. The vallecula, piriform sinuses and perilaryngeal regions should be examined, as well, for pooled secretion of food materials.(9) The presence of candidal or herpetic lesions in the pharynx may indicate similar infection in the esophagus. The presence of erythema and inflammation of the vocal cord may reflect acid reflux disease.
Neck examination should be performed looking for masses, lymphadenopathy or a goiter. Sometimes, a pharyngeal pouch can be felt and a gurgle with regurgitation noted upon pressing on it, indicative of an esophageal diverticulum. The integrity of the larynx and hyoid bone can be assessed by placing the index and middle fingers lightly on each cartilage and observing the vertical laryngeal movement during swallowing. It is useful to observe the patient drinking water, as cough or nasal regurgitation occurs in some cases of oropharyngeal dysphagia. It should be mentioned, however, that, contrary to common belief, the gag reflex does not predict pharyngeal dysfunction or aspiration risk. The gag reflex is actually absent in 20-40% of healthy adults,(10) especially in older persons. A good neurologic examination, including a detailed cranial nerve examination, is essential.
The rest of the physical examination may elicit signs of a certain disease accompanied by dysphagia. For example, tremors and muscular rigidity may indicate Parkinson's, muscular weakness or fasciculation may be a sign of motor neuron disease and evidence of connective tissue disease may indicate scleroderma.
Laboratory Tests
These can lead to the diagnosis of medical conditions that can produce dysphagia. Tests such as anti acetylcholine antibodies (to diagnose myasthenia gravis), muscular enzymes (to diagnose myositis), thyroid function tests (to diagnose thyroid abnormalities) may be ordered when suggested by history and physical.
Diagnostic Techniques
Based on the findings of the history and physical examination, an impression is made regarding the possible type and location of the dysphagia (see algorithm above). This determination will lead to the choice of the initial diagnostic modality, which is usually one of the following: Video Fluoroscopic Swallowing Study (VFSS), barium-contrast esophagogram, upper endoscopy or manometry.
Video Fluoroscopic Swallowing Study (VFSS)
This technique, also known as "modified barium swallow", is the "gold standard" for diagnosing oropharyngeal dysphagia.(11) It is a dynamic test in which the patient is asked to swallow a variety of food items of different consistencies covered with barium. A video fluoroscopic recording is made in both antero-posterior and lateral views. VFSS allows for observation of bolus progress throughout the different stages of the swallowing process. The presence of pooling, delayed transit and laryngeal aspiration can be detected. VFSS allows a slow motion review and assessment of still-pictures. The dynamic nature of this study provides an opportunity to evaluate the response to certain correctional techniques (e.g., chin tucking) during the study. This technique requires the cooperation of an alert patient, which is the most limiting factor to performing VFSS.
When VFSS is not possible because of a high risk of aspiration or if the patient can not be transported to the fluoroscopy suite, other studies are suggested.
Video Endoscopic Swallowing Study (VESS)
VESS is utilized for direct visualization of the oropharynx in action with and without swallowing, using a fiberoptic scope inserted nasally.(12) The fiberoptic scope is placed first above the soft palate to ensure a panoramic view, then at the level of the epiglottis for a better evaluation of aspiration. The addition of methylene blue water may improve the sensitivity of this test. This test is valuable when VFSS can not be performed and is usually done by an otolaryngologist.
Oropharyngeal Manometry
Oropharyngeal manometry is considered difficult to perform because of the rapidly changing pressures in the pharyngeal area and the low tolerance to the placement of the catheter. Newer computerized techniques, utilizing thinner catheters, can provide invaluable information to diagnose UES abnormalities. Performing videofluoroscopy, concurrently with manometry, is known as 'manofluorography' and provides additive information if done properly.(13)
Other techniques available to evaluate oropharyngeal dysphagia, but less practically used, include ultrasonographic evaluation of the laryngeal and hyoid motion,(14) electromyography of individual pharyngeal muscles(15) and scintigraphic bolus analysis to evaluate oral aspiration.(16)
Barium-Contrast Esophagogram (Barium Swallow)
Barium swallow is the initial recommended test if esophageal dysphagia is suspected. The patient is asked to drink liquid barium (and, sometimes. barium coated marshmallows) while pictures are taken in both upright and supine positions. Barium-contrast esophagogram identifies most cases of mechanical obstruction, such as strictures, rings and webs. It can also reveal signs suggestive of a motility abnormality in the esophagus, such as esophageal spasm (corkscrew appearance)(17) or achalasia (bird's-beak appearance).(18)
Esophagoscopy
Esophagoscopy has become the major tool in the evaluation of dysphagia. It is superior to barium swallow for evaluating small lesions of the mucosa and the sequelae of acid reflux disease. For the diagnosis of intramural lesions and extrinsic obstruction, the technique is complementary. For the diagnosis of motility disorders, it is of no value, except when loss of peristalsis is noted during endoscopy, prompting further investigation. Endoscopy provides the opportunity for therapeutic intervention if necessary -- dilatation of strictures using pneumatic balloons or dilators, obtaining a biopsy and removal of foreign bodies.
Esophageal Manometry
This technique is based on the principle of recording pressures throughout the esophageal lumen using a solid-state or perfusion technique. It gives a recording of the peristaltic contractions in the esophageal body including its duration, velocity and amplitude. Normal manometry reveals the presence of sequential esophageal peristalsis during swallowing, the presence of high pressure UES and LES zones at rest that relax completely during swallowing. Manometry is indicated when an esophageal etiology for dysphagia is suspected despite inconclusive barium swallow and endoscopic evaluation.
Different diseases show, on manometry, specific patterns. The presence of simultaneous high amplitude esophageal contractions may represent esophageal spasm. The lack of peristaltic movement with very high LES pressure that does not decrease during swallowing suggests achalasia. It should be noted, however, that as many as one third of patients with achalasia may have a normal LES pressure. A low LES pressure with decreased peristalsis may suggest scleroderma. If simple direct manometry is not revealing, pharmacological provocation, with an agent such as edrophonioum, may be indicated to increase sensitivity.
Treatment
Oropharyngeal Dysphagia
If a reversible or treatable cause of oropharyngeal dysphagia is identified, it should be corrected. This includes cases of thyroid disease, myasthenia gravis and other potentially treatable conditions. Patients with structural lesions (e.g., tumor or Zenker's) should be referred for surgery. In patients with dysphagia, there is a need to identify whether or not the patient can obtain adequate nutrition through the oral route and how high the risk of aspiration pneumonia appears to be. When the ability to obtain adequate nutrition is limited or the aspiration risk is high, non-oral feeding, usually through a gastrostomy tube, should be considered. It needs to be recognized that there is no proof that gastrostomy feeding reduces aspiration risk. If aspiration risk is high, a tracheostomy may also be needed.
Surgical treatments, such as cricopharyngeal myotomy, have been successful in up to 60% of cases but their use remains controversial.
Numerous swallowing therapy techniques have been developed. In general, they can be divided into:
- Dietary modifications
- Thickened liquids when tongue function is disordered or laryngeal closure is impaired.
- Thin liquids are used for weak pharyngeal contraction and reduced cricopharyngeal opening.
- Swallowing maneuvers
- Postural adjustments
- Facilitatory techniques, such as strengthening exercises, biofeedback, thermal and gustatory stimulation.
As concluded in a recent review in Gastroenterology, "management of oropharyngeal dysphagia is currently an inexact science."(22)
Dysphagia of the Distal Esophagus
In general, management of dysphagia, secondary to problems in the distal esophagus, is dependent on the endoscopy findings and, in the case of achalasia, on the results of manometry.
When no lesion is present, the treatment of choice for esophageal motility disorders is cisapride or domperidone. Other prokinetic agents are under development. If these agents fail, dilatation to between 40 F and 54 F should be undertaken. Reflux esophagus is first treated with anti-reflux therapy (proton pump inhibitors, cisapride or domperidone and, when indicated, funduplication) and, if that fails, dilation. Infections should be treated with the appropriate chemotherapy, e.g., nystatin swallows for candida. Tumors require surgery and pseudoachalasia associated with tumors needs to be identified.
Patients with achalasia, who are good operative risks, should receive surgery. The preferred operation is laparoscopic myotomy. Pneumatic dilation, once an acceptable treatment modality, is rarely used now because of the risk of perforation. If the patient is a poor operative risk, medical therapy with nitrates and/or calcium channel blockers can be utilized. If this is unsuccessful, bougienage [passing flexible, cylindrical dilators (bougies)] of increasing diameter into the esophagus and across the area of constriction) using a 45 F to 60 F dilator can be tried. Other therapies include injection with botulinium toxin, myotomy or pneumatic dilatation.
Management of a simple peptic stricture of the esophagus (diameter >10 mm and not tortuous) consists of progressive dilation and anti-reflux therapy. Complex strictures are treated the same way but mercury bougies can not be used. If dysphagia returns, repeated dilation, including self-bougienage, can be used. Other options include steroid injection and surgery. Persons with a lower esophageal ring (Schatzki's ring) require dilation and treatment for reflux esophagitis and/or motility problems if they co-exist.
Management options for different conditions causing esophageal dysphagia are summarized in Table 2.
Table 2. Management Options for Esophageal Dysphagia.
| Condition | Conservative treatment | Invasive treatment |
|
|
|
The American Gastroenterology Association has recently published position statements and associated in-depth reviews on oropharyngeal (February 1999 issue of Gastroenterology) and esophageal (July 1999 issue of Gastroenterology) dysphagia. These are highly recommended to those interested in more information concerning dysphagia.