Course Authors
A. Iain McGhie, M.D.
Dr. McGhie is Associate Professor of Medicine, Director, Exercise and Nuclear Cardiology Laboratories at the University of Texas - Houston Medical School Department of Internal Medicine (Cardiology Division).
Dr. McGhie reports no commercial conflict of interest.
This activity is made possible by an unrestricted educational grant from the Novartis Foundation for Gerontology.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the different modalities of stress that are available for use in conjunction with myocardial perfusion imaging
List the different radiopharmaceuticals that are available for myocardial perfusion imaging and understand their use
Describe the basic physiologic principles of stress myocardial perfusion imaging
Apply stress myocardial perfusion imaging in clinical practice.
In clinical practice, there are many ways to evaluate and risk stratify those with known coronary disease. The cardiac nuclear imaging techniques can be very useful but there has been so much evolution in the techniques recently that the variety of tests and their optimal use can be elusive and intimidating. Dr. Iain McGhie, in this issue of Cyberounds® Cardiovascular, provides us with an excellent review of new imaging techniques for the evaluation of patients with known or suspected coronary disease. Dr. McGhie is an expert nuclear cardiologist and a gifted teacher. Please welcome him to Cyberounds®!
-- Richard W. Smalling, M.D., Ph.D., Cardiovascular Moderator
Background
Approximately three to four million stress nuclear myocardial perfusion imaging (MPI) studies are performed annually in the United States. There have been many developments in this technology since the technique was first introduced approximately two decades ago. This monograph has been written to provide a guide to the selection of stress nuclear tests for general practitioners and internists. It provides an overview of MPI for patients with suspected or known coronary artery disease. The first section deals with the different forms of stress modalities available. The second section deals with the different radiopharmaceuticals that are available for stress MPI. The third section describes the most commonly used MPI protocols. The fourth section gives a summary of the clinical application of stress testing for patients with known or suspected coronary artery disease.
There are several reasons for selecting MPI over regular "vanilla" ECG stress testing. First, the presence of ECG abnormalities on the baseline ECG may prevent interpretation of changes that occur during exercise, e.g., marked ST-T changes, left ventricular hypertrophy, bundle branch block, digoxin administration and ventricular pre-excitation. Second, certain patient populations have a high incidence of false positive exercise ECGs, e.g., premenopausal women and patients with mitral valve prolapse. Third, MPI offers a higher degree of accuracy and greater prognostic power, which may be important, in certain patients, e.g., patients with known coronary artery disease. Finally, patients who are unable to exercise and are undergoing pharmacologic stress testing will require some kind of imaging procedure.
Selection of Stress Modality
Exercise
The cardiovascular response to dynamic exercise is an increase in cardiac output. The peripheral resistance decreases in active muscles and increases in resting tissues with a resultant fall in systemic vascular resistance. There is an increase in heart rate in response to exercise, mediated by alterations in the autonomic nervous system. The increase is linearly related to work load and oxygen uptake. The increases in cardiac output during dynamic exercise increase systolic arterial blood pressure with little alteration in the diastolic pressure.
For many patients, exercise is the preferred means for stress,(1) in large part because of the additional prognostic information it provides in patients with coronary artery disease. In general terms, the severity of CAD correlates with the type of ST-segment depression (downward > horizontal), and with the amount, time of appearance, duration and number of leads with ST segment depression.(2) In addition, when ST-segment depression occurs at a lower work load or at a lower pressure-rate product, i.e., HR x SBP, the poorer the prognosis and the more likely multi-vessel disease is present. The persistence of ST depression during the recovery phase is also an indicator of more severe and extensive CAD.
Pharmacologic stress
Increasingly, pharmacologic modalities are being employed for stress testing, with the main indication being the patient's inability to exercise adequately. Reasons for this are varied and include concomitant medical conditions, e.g., peripheral vascular disease, morbid obesity and neurologic disease; poor motivation; concomitant anti-anginal medication (in particular, b-adrenergic and calcium channel antagonists).
Dipyridamole is the most widely used pharmacologic agent for use with myocardial perfusion imaging agents and has a similar diagnostic accuracy as exercise MPI.(3) Dipyridamole causes an increase in endogenous adenosine levels by blocking uptake of adenosine by red cells and endothelium. Increased concentrations of adenosine in the interstitial fluid results in relaxation of vascular smooth muscle, including the coronary arterioles. Once adenosine leaves the interstitium, it undergoes rapid intracellular metabolism via adenosine kinase by phosphorylation or deamination by adenosine deaminase.
Patients should be in a fasting state and should not have taken any xanthine medications (theophylline) in the previous 36 hours and caffeine beverages (including decaffeinated coffee, tea and cola) in the preceding 24 hours. Patients should be in a stable and compensated condition with no history of recent unstable angina or complicated acute myocardial infarction. It is contraindicated in patients with reversible airway obstruction. The infusion of 0.142 mg/kg/min of dipyridamole is given over four minutes with the radioisotope being administered three to four minutes later.
Dipyridamole may alter systemic hemodynamics, with a slight fall in blood pressure and slight increases in the heart rate and pressure-rate product. Side effects are reported in approximately 50% of patients.(4) These include chest pain that occurs in approximately 15%-40% and ST segment depression in 5%-20% of patients. The presence of either or both does not reliably predict the presence of angiographically significant disease, although it is more common in their presence. Non-cardiac symptoms are relatively common and include flushing, nausea, lightheadedness and mild headaches. Serious adverse reactions are rare following intravenous dipyridamole but fatal and non-fatal myocardial infarction, ventricular fibrillation and tachycardia, transient cerebral ischemia and bronchospasm have been reported. The effects of dipyridamole are usually rapidly reversed by the administration of aminophylline which antagonizes the effects of adenosine at the A2 receptor.
Adenosine, the mediator of dipyridamole's vasodilating action, can also be used as a coronary vasodilator.(5) Maximal coronary vasodilatation is obtained with an intravenous infusion rate of 100-140µg/kg/min. The effects are maximal two minutes after the onset of the infusion and return to baseline within two to three minutes of its discontinuation. Adenosine has a comparable diagnostic accuracy to dipyridamole. Like dipyridamole, adenosine results in small but significant increases in heart rate and falls in the systolic and diastolic blood pressures. Side effects are more frequent with adenosine but are generally better tolerated. Common side effects include chest pain, dyspnea, flushing, headache, ST segment depression and first and second degree AV block. In the majority of patients, the side effects ceased rapidly after terminating the adenosine infusion.(6)
Dobutamine is a potent sympathomimetic agent with stimulatory effects on b1, b2 and a-adrenoreceptors with predominantly inotropic and a lesser chronotropic effects.(7),(8),(9),(10) Therefore, dobutamine produces an increase in myocardial oxygen requirement by causing an increase in myocardial contractility and systolic blood pressure and, at higher doses, an increase in heart rate. The dose for dobutamine is usually 10 µg/kg/min increased by increments every three minutes to a maximal infusion rate to 40-50 µg/kg/min.
Side effects that can occur with this agent include palpitation, headache, paresthesia, nausea, tremor, ventricular arrhythmia and marked ST segment depression. Side effects usually resolve following discontinuation of the infusion but may require use of intravenous B-blockers for reversal of side effects. Experimental evidence suggests that dipyridamole produces greater increase in coronary flow rates than dobutamine, making the former the pharmacologic agent of choice in stress myocardial perfusion imaging. At present, the main role of dobutamine in MPI is when the use of dipyridamole or adenosine is contraindicated, usually in patients with reversible obstructive airways disease.
Choice of Radiopharmaceutical
Physiology of Perfusion Imaging
The basic underlying physiological principles of myocardial perfusion imaging is as follows. At rest, coronary flow is normal even in the presence of a narrowing of up to 85% diameter stenosis.(11) Stress produces an increase in coronary flow - in a normal coronary artery, flow increases 2-2.5 fold with dynamic exercise or by 3-4 fold with maximal coronary vasodilation, i.e., with adenosine or dipyridamole. However, in a stenosed artery, the increase in flow is attenuated, with an increase in the pressure gradient across the stenosis, resulting in a drop in pressure and flow distal to the stenosis. This causes a heterogeneous distribution of blood flow during stress, with a greater increase in myocardial perfusion in the area subtended by the normal coronary artery relative to the myocardium supplied by the stenotic artery. Therefore, a lesser amount of the radiopharmaceutical injected during stress will be distributed to the myocardium supplied by the stenosed coronary artery resulting in the appearance of a perfusion defect.
Thallium-201
This cation is a potassium analog and has been widely used in MPI.(12) Myocardium uptake occurs by both passive and active mechanisms involving Na,K-ATPase.(13) There is a linear relationship between Tl-201 uptake and coronary blood flow, with a tendency to underestimate and to overestimate at the upper and lower limits of the physiologic range, respectively. Thallium washes out of the myocardium by diffusion with a half-life in the myocardium of 4-8 hours. The rate is primarily dependent on the Tl-201concentration gradient between the myocardium and blood.
During stress, the coronary flow rates in stenosed arteries are lower than those achieved in normal coronary arteries. Consequently, there is less Tl-201 uptake by the myocardium in the distribution of the stenosed coronary arteries. Following stress, coronary flow returns to baseline levels and is the same in both the normal and stenosed arteries. However, because of the unequal distribution of Tl-201 in the myocardium during stress, clearance of Tl-201 is heterogeneous. The greater concentration gradient between the normal myocardium and blood results in a higher washout rate of Tl-201 than in the hypoperfused myocardium where the Tl-201 concentration gradient is less. Therefore, there is a trend for the myocardial concentration of Tl-201 to equalize with time in the normal and abnormally perfused myocardium. Thus the apparent "redistribution" of Tl-201, with the stress-induced perfusion defect apparently reversing during rest imaging three to four hours later. A perfusion defect following stress that has resolved by time of distribution imaging is considered to represent ischemic but viable myocardium. Conversely, a defect present at time of redistribution imaging was classically interpreted as representing non-viable, scar tissue. However, several studies have shown that detection of reversibility can be enhanced by re-injection of Tl-201 imaging in the presence of a severe perfusion defect at the time of 4-hour redistribution imaging.(14),(15)
Technetium-99m SestaMIBI (Cardiolite®)
99mTc-labeled radiopharmaceuticals were developed in the early 1980's.(16) In comparison to Tl-201, Tc-99m labeled radiopharmaceuticals offer significant advantages.(17),(18) This includes a shorter half-life (t1/2 = 6 hours) allowing a larger patient dose to be administered, typically 25 - 35 mCi compared to 3 - 4 mCi of Tl-201. In addition, the higher energy of the Tc-99m, 140 keV versus 74 keV for Tl-201, provides superior imaging characteristics. Tc-99m sestaMIBI is a lipophilic cation that accumulates in the myocardium according to blood flow, although, at higher coronary flow rates, the relationship becomes non-linear, resulting in an underestimation of coronary flow. Uptake occurs primarily by diffusion, promoted by negative electrical gradients across sarcolemmal and inner mitochondrial membranes and to lesser extent concentration gradients. Unlike Tl-201, Tc-99m sestaMIBI does not undergo significant redistribution.
Clinical studies to date indicate that imaging with Tc-99m sestaMIBI have demonstrated a similar degree of accuracy for the overall detection of coronary artery disease.(19),(20) In addition, it produces higher quality images and is more accurate for the detection of individual coronary artery stenoses. The higher count rates and lack of redistribution also allow gated tomographic acquisitions to be performed, enabling evaluation of regional and global ventricular function.(21),(22),(23)
Technetium-99m Tetrofosmin (Myoview®)
Tetrofosmin is another cationic lipophilic Tc-99m labeled radiopharmaceutical that has characteristics similar to Tc-99m sestaMIBI. Several clinical trials have shown it to provide similar diagnostic accuracy as Tl-201 imaging.
Imaging Protocols
The choice of different forms of stress and radiopharmaceuticals has resulted in large number of different imaging protocols.(24)
Tl-201 imaging protocols: The kinetics of Tl-201 mandate that stress imaging is performed within 10 - 15 minutes following completion of exercise. Following acquisition of the stress images, redistribution imaging is performed three to four hours later. If a severe perfusion defect is present after redistribution, imaging re-injection is typically performed.
Tc-99m SestaMIBI (Cardiolite®) Imaging Protocols
Lack of significant redistribution of Tc-99m sestaMIBI has led to the development of different imaging protocols. The most widely used is the same-day rest-stress protocol. Using this protocol, 8 - 9 mCi of Tc-99m sestaMIBI are injected at rest and imaging performed approximately one hour later to allow for clearance of splanchnic, in particular, hepatic activity. After acquisition of the resting images, the patient is prepared for stress, which can be performed after a minimal interval of one hour following the resting injection. At peak stress, 25 - 30 mCi of Tc-99m sestaMIBI are injected, with imaging being performed 15 - 60 minutes later. Typically, imaging can be performed 15 minutes after exercise; however, if pharmacologic stress is used, imaging is delayed for approximately 60 minutes to allow time for splanchnic activity to decrease and,therefore, improve image quality. The use of the large dose of Tc-99m sestaMIBI for the stress injection has the advantage that stress images are of the highest quality and allows for acquisition of ECG-gated SPECT images.
An alternative protocol is to perform the stress images first using the lower 8 - 9 mCi dose of Tc-99m sestaMIBI followed by the resting images using 25 - 30 mCi Tc-99m sestaMIBI. Similar protocols are used for Tc-99m tetrofosmin (Myoview®).
Dual Isotope Protocols
Dual isotope protocols have become increasingly popular over the last few years(25) because the studies are generally faster and, in addition, allow for the opportunity to perform Tl-201 rest-redistribution imaging when viability is a concern. Typically, 2.5 mCi of Tl-201 are injected at rest and images are acquired 15 minutes later. This is immediately followed by stress perfusion imaging with Tc-99m sestaMIBI (25 mCi).
Clinical Applications
Diagnosis of Coronary Artery Disease
Stress MPI has a very important role in the diagnosis and evaluation of patients with suspected and known coronary artery disease. Over 3,000,000 stress myocardial perfusion studies are performed each year in the U.S. Imaging is performed using either planar or tomographic techniques; however, the latter technique, single photon emission computed tomography (SPECT), is generally preferred -- SPECT has greater accuracy and superior localization abilities, particularly in the identification of left circumflex coronary disease and multi-vessel coronary disease.(26) The sensitivity and specificity of SPECT imaging is in the region of 85 - 88%.(27),(28),(29),(30),(31)

Figure 2. Figure Headline.
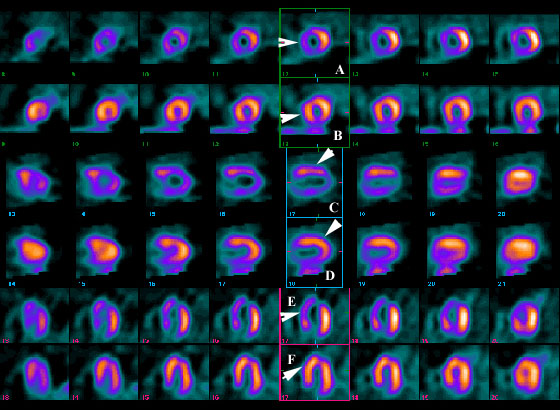
Click to see full sized image
These images represent a typical appearance of reduced relative myocardial isotope uptake in the region supplied by the left anterior descending coronary artery (arrows) during stress (frames A, C and E) with normalization of relative uptake at rest (frames B, D and F). This pattern is consistent with a high-grade proximal LAD lesion supplying a bed of viable (non-infarcted) myocardium.

Evaluation of Patients with Suspected or Known Coronary Artery Disease
Stress myocardial perfusion imaging is also a powerful prognostic technique for risk-stratifying patients with known or suspected coronary artery disease.(32),(33) The number of reversible perfusion defects was the best predictor of future cardiac events. Findings fromTI-201 imaging were found to have more predictive value than clinical, exercise or ECG data. Studies that have included angiographic data reveal that scintigraphic findings still provide important and independent prognostic data. In addition to the number and severity of perfusion defects, the amount of Tl-201 uptake by the lungs and the presence of transient dilatation of the left ventricle following exercise have also been found to be associated with an adverse prognosis.(34)
Patients with chest pain, with known or suspected coronary artery disease, who have normal MPI have an excellent prognosis. Cardiac event rates in patients with a normal SPECT perfusion study are very low. In a study of 1,926 patients, the annual event rate of < 0.42%/year in patients with normal SPECT Tl-201 images compared with 2.1%/year in patients with an abnormal 201Tl images (p<0.001).(35) Similar data are also available for exercise and dipyridamole sestaMIBI imaging.(36),(37)
The paradigm for using stress MPI to risk-stratify patients is that patients with low-risk studies can be managed more conservatively with medical therapy and aggressive risk-factor modification. Patients who have high risk findings on stress MPI can be considered for revascularization and undergo coronary arteriography.(38) Even after the most powerful clinical and exercise variables are used to predict prognosis, the addition of the nuclear information provided important incremental (five-fold increase) prognostic information in all patient subgroups.
Evaluation Following Acute Coronary Syndromes
Many clinical and laboratory variables have been used to identify survivors of myocardial infarction that are at high risk of future cardiac events. These include recurrent angina, clinical left heart failure, depressed left ventricular ejection fraction and complex ventricular arrhythmia.(39),(40),(41) Exercise electrocardiography can provide important prognostic information following acute myocardial infarction.(42),(43) However, stress myocardial perfusion imaging offer several advantages. These advantages include higher sensitivity and specificity for the detection of multivessel disease in infarct survivors, ability to localize ischemia to particular coronary distributions and the ability to detect ischemia in the infarct zone, as well as the non-infarct zone.
Gibson et al.(44) found that perfusion defects that redistributed and defects that involved multiple vascular territories or patients with increased Tl-201 lung uptake were associated with an adverse prognosis. They also showed that stratification of patients into low and high risk groups, based on the MPI findings, had greater discrimination than either exercise electrocardiographic or angiographic data. More recently, Dakik et al.(45) followed 71 patients with acute MI treated with thrombolytic therapy over 26 months and found that by multivariate analysis the significant predictors of risk were ejection fraction (P <0.0005) and the size of the ischemic perfusion defect (P = 0.005). They found the combination of ejection fraction and SPECT Tl-201 findings added significant incremental prognostic information to the clinical data, whereas angiography did not further improve a model that included clinical, ejection fraction and SPECT Tl-201 variables.
Dipyridamole stress has also been used successfully in patients following a recent myocardial infarction.(46),(47),(48) It has the advantage of producing greater increases in coronary flow than submaximal exercise, with only minor increases in myocardial O2 requirements; in addition, its effects can be rapidly reversed by intravenous aminophylline. Brown et al. used dipyridamole Tl-201 early (1-4 days) following infarction with no adverse events.(49) The presence of 201Tl redistribution in the infarct-zone (present in 45% of patients) was the only predictor of in-hospital events (p=0.001). None of the angiographic variables was significant predictors of cardiac events. Similar findings have been published using adenosine Tl-201 SPECT.(50)
Stress MPI also has a role to play in the evaluation of patients with unstable angina. The presence of reversible perfusion defects is an important independent predictor of future adverse cardiac events in patents with unstable angina who have been stabilized with medical therapy.(51),(52) Recently published guidelines support the use of stress perfusion imaging for risk stratification in low or intermediate risk patients.(53)
Preoperative Assessment for Non-Cardiac Surgery
Dipyridamole or adenosine MPI has been successfully used in the preoperative assessment of patients undergoing elective reconstructive vascular surgery.(54),(55),(56),(57),(58),(59) These patients have a high probability of concomitant coronary artery disease but cannot perform adequately for exercise testing. Patients with a normal or mildly abnormal Tl-201 or those with a fixed Tl-201 perfusion defect have a low perioperative event rate. Eagle et al.(59) showed that MPI is best utilized when patients are first risk stratified using clinical and ECG findings. The presence of Q waves, ventricular ectopy, diabetes mellitus, advanced age and angina were all associated with increased risk. Patients that have three or more of these risk factors have a high cardiac event rate of 50% and may warrant undergoing coronary arteriography directly. While patients with one to two clinical markers (intermediate risk) can be risk stratified by MPI, patients with no risk factors had a low risk and no benefit was observed from performing MPI imaging. Guidelines for perioperative evaluation of patients undergoing non-cardiac surgery have recently been published.(60)
Assessment of Re-vascularization Procedures
Stress myocardial perfusion imaging is of value in evaluating patients prior to revascularization procedures, in particular, coronary angioplasty.(1) It can be used to demonstrate the presence of a stress-induced perfusion defect associated with a particular coronary artery stenosis, thus allowing targeting of the "culprit lesion" for angioplasty. Following angioplasty, MPI can be used to evaluate the adequacy of revascularization and also to predict restenosis - however, timing of MPI following angioplasty is important and should be delayed for four to six weeks.(61)
Several studies have found a poor correlation between angiographic results and MPI when performed earlier than four to six weeks following angioplasty, with the presence of areas of hypoperfusion, despite good angiographic results. These perfusion abnormalities are transient, usually resolving within one to two months of an angiographically successful dilatation. When delayed for four to six weeks, patients with normal MPI have a very low restenosis rate compared to those with abnormal MPI who have a 85% and 96% restenosis rate after six and 12 months, respectively. MPI is also of value in evaluating patients following coronary artery bypass surgery and is generally superior to exercise electrocardiography in determining the status of graft patency.(62),(63),(64)