Course Authors
Robert J. Pignolo, Jr., M.D., Ph.D. is Professor of Medicine and Chair, Department of Internal Medicine, Division of Geriatric Medicine and Gerontology, Mayo Clinic Alix School of Medicine, Rochester, MN.
Updated by Jessica Shu, M.D., Ben Alencherry, M.D., and Aman Rajpal, M.D.
Dr. Shu is Chief Resident and Dr. Rajpal is Assistant Professor, Department of Medicine, Division of Clinical and Molecular Endocrinology, Case Western Reserve University and Louis Stokes Cleveland VA Medical Center. Dr. Alencherry is Cardiology Fellow, The Cleveland Clinic.
Within the past 12 months, Drs. Shu, Alencherry and Rajpal report no commercial conflicts of interest. Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose relevant to this activity.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Osteoporosis is a disorder characterized by reduced bone mass and microarchitectural deterioration of bone tissue that results in an increase in bone fragility and susceptibility to fracture. Osteoporosis affects both men and women and is a preventable disease. There are often multiple etiologies or risk factors for bone loss and these underlying secondary causes should always be addressed.
Epidemiology
In 2010, 10.3% of adults or 10.2 million Americans of age 50 or older had osteoporosis. (1) As per National Osteoporosis Foundation (NOF), Osteoporosis is responsible for an estimated two million fractures per year, yet nearly 80% of older Americans who suffer fractures are not tested or treated for osteoporosis. Osteoporosis is an expensive disease, as osteoporosis-related fractures cost around $19 billion to the healthcare system annually. In an observational study by the Women's Health Initiative, the fracture incidence in non-Black women over one year was greater than the incidence of invasive breast cancer and cardiovascular disease events combined. (2)
Individuals with fractures may experience pain, dependence, depression and skeletal deformity. (3) Patients with hip fractures were found to have longer hospital stays and were more likely to require rehabilitation afterwards. Only ~40% of hip fracture survivors are able to return to their prior level of activities of daily living (ADLs), and even fewer (~25%) return to their pre-fracture level for instrumental ADLs. Osteoporotic fractures are associated with increased mortality; one-year mortality rate is 12-36%. (4)(5) Up to 90-95% of fractures in hospitalized patients >60 years are attributable to osteoporosis.(6)(7) Currently <15% of those with fragility fractures are evaluated and treated for osteoporosis despite a 1.5 to 9.5-fold increased risk of future fracture.(6)(8)(9)
Although important steps have been initiated in advancing efforts to diagnose and treat osteoporosis and to reduce barriers that prevent diagnosis and treatment, (8)(10)(11)(12) there remain many patients who are evaluated for bone loss for the first time with either advanced disease or at the time of fracture.(13)(14)(15)(16)
There are many barriers to initiating treatment for patients who have or are at risk for osteoporosis and fractures. (12) Lack of patient and primary care physicians' knowledge, lack of awareness and the perception by orthopedic surgeons that evaluation and treatment of osteoporosis is not their responsibility may all pose difficult challenges. The cost of therapy, time and cost of diagnosing osteoporosis, fear of possible side effects of medications, as well as confusion about medications or their effectiveness, may also account for failure to adequately treat patients with osteoporosis.
Other potential barriers include complex medical conditions in elderly patients, reluctance of elderly patients to add more medications, lack of access to BMD testing and inadequate time to address secondary prevention. Fear of possible side effects appears to be the most common reason for lack of initiation or discontinuation of osteoporosis medications. (17)
Risk Factors
Risk factors for osteoporosis can be broadly classified as:
- Modifiable: Gonadal deficiency, anorexia nervosa, Vitamin D deficiency, exposure to certain medications (such as glucocorticoids and some anticonvulsants), sedentary lifestyle, cigarette smoking, alcohol intake;
- Non-modifiable: Age, sex, BMI, ethnicity, family history.
Screening
Current guidelines recommend screening for:
- All postmenopausal women, age >65 years;
- All men age >70 years;
- Postmenopausal women <65 years with clinical risk factors for fracture (previous non-traumatic fracture, glucocorticoid therapy, parental history of hip fracture, low body weight, current cigarette smoking, excessive alcohol consumption (>3 drinks/day), rheumatoid arthritis, known comorbidity causing secondary osteoporosis);
- Delayed puberty;
- Hypogonadism in men;
- Early menopause in women;
- Known comorbidity causing secondary osteoporosis (see Table 1 below).
General Approach to Evaluating Patients With Osteoporosis and Osteoporotic Fractures
All patients with or at risk for osteoporosis should have a complete skeletal history, risk factor assessment, physical examination (including fall assessment), laboratory studies to rule out possible secondary causes of bone loss, and a measurement of bone mineral density (BMD). The approach to the patient with an osteoporotic fracture incorporates these same basic clinical principles, but with the added intention to reduce their high future morbidity and mortality as well as to preserve function.
Skeletal History
A complete skeletal history should include documentation of previous fracture – how they were sustained (traumatic versus minimal trauma) and if delayed fracture healing occurred. Multiple falls, even in the absence of previous fracture, should be carefully explored for potential mechanisms. Previous diagnosis of osteoporosis, time since initial diagnosis, and previous or current treatments should be obtained. Other information from the skeletal history should include known bone deformities, complaints of bone or musculoskeletal pain, reduced mobility, height loss and current primary bone disorders. Other details related to fracture risk is formally obtained by a risk factor assessment.
Risk Factor Assessment
The assessment of risk for bone loss and fractures can be divided into categories of personal risk factors, secondary medical conditions that cause bone loss and medications that adversely affect mineral metabolism and/or bone turnover. (19) (20)
Non-modifiable personal risk factors associated with osteoporosis include a family history of osteoporosis or fracture, low calorie intake or calcium/vitamin D deficient diet during formative years, personal history of fracture as an adult, increasing age, early menopause (<45 years old), late menarche (>16 years old), thin body frame, history of amenorrhea or irregular menstrual periods, female sex, white (and perhaps Asian) ancestry, history of prolonged periods of bed rest (or immobilization) and tallness.
As reflected by their inclusion in multiple algorithms that predict the need for bone mineral density (BMD) testing, age and body weight, as well as lifetime exposure to estrogen in women, are among the more important personal risk factors. (21)(22)(23)(24)(25)(26)
Osteoporosis and fractures are less common in men due to their larger skeletons, bone loss starting later in life, slower progression, and the absence of a rapid phase of bone loss as occurs in menopause; however, men have much higher mortality and chronic disability rates after a hip fracture. (27) They are also more likely to have a secondary cause of bone loss compared to women.
Potentially modifiable personal risk factors associated with osteoporosis and/or fracture are low body weight, low calorie intake or calcium/vitamin D deficient diet, sedentary lifestyle or inadequate physical activity, heavy alcohol use (three or more drinks/day), high salt intake, tobacco cigarette smoking (active or passive) and high caffeine intake. (19)(20) Independent risk factors for fractures include impaired neuromuscular function, decreased visual acuity, sedative/hypnotic drug use, and frequent falls, previous fractures and low body weight.
Secondary Medical Conditions
As outlined in Table 1, a number of co-existing or past medical conditions can predispose individuals to continued bone loss. (28) Elderly patients are unlikely to present with genetic conditions that otherwise would present much earlier in life or limit life expectancy. However, there are predisposing medical conditions that are more likely to contribute to age-related bone loss and fracture such as vitamin D deficiency, post-menopausal status and chronic kidney disease.

Table 1. Secondary Medical Conditions that Alter Bone Turnover and Mineral Homeostasis.
| Endocrine | Hyperthyroidism, hyperparathyroidism, Cushing's syndrome, diabetes mellitus, hyperprolactinemia, hypogonadism, adrenal insufficiency, athletic amenorrhea, premature menopause, pregnancy and lactation |
| Rheumatologic | Rheumatoid arthritis, ankylosing spondylitis, sarcoidosis, lupus |
| Gastrointestinal/Malabsorption | Chronic liver disease, hepatobiliary dysfunction, vitamin D nutritional deficiency, calcium deficiency, parenteral nutrition, malabsorption syndromes (celiac disease, gastric bypass, inflammatory bowel disease, pancreatic disease, short bowel syndrome) |
| Hematological/Oncological | Mastocytosis, hemolytic anemia, malignancy (general), multiple myeloma, hemophilia, thalassemia, leukemia, lymphomas |
| Renal | Idiopathic hypercalciuria (on low calcium diet), CKD/renal osteodystrophy, renal tubular acidosis |
| Psychiatric | Eating disorders (anorexia, bulimia), depression |
| Other | Alcoholism, Paget's disease, osteogenesis imperfecta, amyloidosis, epidermolysis bullosa, hemochromotosis, multiple sclerosis, chronic metabolic acidosis, congestive heart failure, COPD, emphysema, seizure disorder, idiopathic scoliosis, muscular dystrophy, post-transplant bone disease, HIV/AIDS, weight loss |
More common conditions are bolded.

Medications that Adversely Affect Mineral Homeostasis or Bone Turnover
Bone loss due to medications is common, (29)(30) especially in the elderly. However, evidence that supports a clear etiological role for certain medications is variable. A list of medications with well-established contributions to bone loss is shown in Table 2. Glucocorticoid-induced osteoporosis (31) and bone loss due to immunosuppressive therapy after solid organ transplant (32) remain challenging problems.

Table 2. Pharmacologic Causes of Secondary Osteoporosis.
| Glucocorticoids |
| Methotrexate |
| Proton pump inhibitors (chronic use) |
| Thyroid hormone in supraphysiologic doses (especially in the case of TSH suppression in thyroid cancers) |
| Tamoxifen (premenopausal) |
| Aromatase inhibitors |
| Gonadotropin-releasing hormone (agonist or antagonist) |
| Depo-medroxprogesterone |
| SSRIs |
| Lithium |
| Antiepileptic drugs |
| Cytotoxic drugs (chemotherapy) |
| Immunosuppressive therapy |
| Cyclosporin A and Tacrolimus |
| Thiazolidinediones |
| Vitamin A, excessive intake |
| Prolonged anticoagulation (heparin) |
| Megestrol acetate |

Why Is Risk Factor Assessment Important?
As mentioned above, approximately 10 million Americans have osteoporosis, but another 44 million have low bone density or osteopenia, placing them at increased risk of osteoporosis.(1) According to the National Osteoporosis Risk Assessment group most of the fractures occurred in women who did not have osteoporosis. Since fractures happen at all BMD levels, there is, clearly, more to bone strength than bone density. Therefore, combining risk factors with BMD increases the likelihood of predicting the risk of fracture. Low bone density is a strong predictor of fracture risk, but it still does not carry the same weight as other risk factors or combination of risk factors.
A Web-based tool called Fracture Risk Assessment Tool or FRAX uses BMD and a combination of risk factors to predict the likelihood of fractures (10-year risk of a major fracture or hip fracture), but may have limited value in clinical practice. Some country-specific FRAX algorithms are available online.
Risk factors used in the FRAX algorithm includes prior fracture, age, BMI, femoral neck BMD, family history of hip fracture (parents), corticosteroid use, alcohol intake (>3 drinks/day), smoking (current), rheumatoid arthritis and secondary osteoporosis. The use of FRAX in clinical practice can provide supportive data for decision-making about pharmacological interventions when the decision to treat is in doubt. It is applicable to postmenopausal women and men >50 years old of all racial backgrounds but most appropriate for otherwise healthy patients with stable skeletal status. The National Osteoporosis Foundation (NOF) has promoted the guideline that treatment should be initiated in patients with osteopenia (T-score by DEXA scan between -1 and -2.5) and a 10-year risk of major fracture >20% (or of hip fracture >3%) as calculated by FRAX.
Use of the FRAX algorithm is limited by the paucity of information on treatment efficacy in non-osteoporotic patients, the lack of accounting for BMD at sites other than femoral neck and the failure to consider risk factors for falls. FRAX also defines some risk factors as categorical rather than continuous (e.g., glucocorticoid use), and so makes no distinction between high- or low-dose steroid use (or duration of use). Additionally, FRAX is shown to underestimate the fracture risk in men.
FRAX is not applicable to young adults (<40 years), patients on treatment, situations where rapid bone loss is expected (early menopause, discontinuation of HRT, hormone deprivation therapy, initiation of glucocorticoids) or secondary causes of bone loss. Caucasian fracture rates are based on a small number of patients and non-Caucasian fracture rates are extrapolated from Caucasian rates. Some of these pitfalls are likely to be addressed with future versions of FRAX.
Osteoporosis In Men
Osteoporosis and fractures are less common in men because of their larger skeletons, bone loss starting later in life, slower progression and the absence of a rapid phase of bone loss as occurs in menopause. Men suffer one-fifth to one-third of all hip fractures, and half as many symptomatic vertebral fractures compared to women; however, men have much higher mortality and chronic disability rates after a hip fracture. (27) They are also more likely to have secondary cause(s) of bone loss compared to women.
Physical Exam
The physical examination should focus on detecting the consequences of osteoporosis (fractures), secondary medical causes of bone loss and an initial assessment of fall risk. In the absence of exam findings for vertebral compression fractures or occult nonvertebral fractures, physical signs cannot confirm the diagnosis of osteoporosis. Findings that support occurrence of vertebral fractures include hyperkyphosis (where strict upright posture becomes impossible).(33) This is accompanied by height loss, and with multiple vertebral fractures, narrowed gapping between the ribs and ilium with or without the 12th rib resting on the iliac crest (rib-on-pelvis syndrome). Other suggestive findings may include a protruding abdomen, paravertebral muscle spasm and vertebral tenderness.
Pain usually occurs with acute fracture, but may remain for months after fracture and many patients have chronic pain after vertebral fractures. Hyperkyphosis may be measured by a flexicurve device, radiographically using calculated Cobb angle (>50°) from a standing lateral radiograph, wall-occiput test (wall-occiput distance >0 cm), or rib-pelvis test (rib-iliac crest distance ?2 finger breadths).(33)(34)
Vertebral fractures, however, account for only one cause of hyperkyphosis. Other possibilities include muscle weakness, disc height loss, ligament contraction, physical inactivity, postural changes and heritability. The possible consequences of hyperkyphosis, regardless of etiology, are compromised pulmonary function, poor physical function, falls, osteoporotic fractures, decreased quality of life, depression and increased mortality independent of vertebral fractures. (34)(35) Occult nonvertebral fractures may present with bony tenderness, difficulty with weight bearing, as well as difficulty with joint positioning or movement.
The abundance of medical disorders that can cause bone loss precludes a complete description of exam findings for each condition. Examples of obvious signs to prompt further inquiry include the bony deformities consistent with rheumatoid arthritis, stigmata of chronic alcoholism and liver disease, scars suggesting neck surgery (thyroid or parathyroid), cushingoid appearance, possible decrease in testes size and gynecomastia in hypogonadism, and skin changes associated with specific endocrinopathies.
A minimal fall assessment on physical exam should include the ability to rise from a chair without using the upper extremities, measurement of resting pulse, gross visual testing, walking style and heel-to-toe ambulation. Difficulty in rising from a chair without the use of the arms, a resting pulse >80, poor visual acuity and gait dysfunction, including imbalance, all suggest increased fall risk.
Laboratory Testing
Screening laboratory tests serve to rule out secondary medical causes of bone loss. Normal serum electrolytes, liver and kidney function tests, albumin, total protein, calcium, intact parathyroid hormone (PTH), 25-hydroxy vitamin D, phosphorus, magnesium, thyroid-stimulating hormone (TSH), serum testosterone (in men) and a complete blood count eliminate most secondary causes of bone loss. A 24-hour collection for urinary calcium, sodium and creatinine may also be helpful. A low 24-hour urinary calcium may suggest vitamin D deficiency, osteomalacia or malnutrition (e.g., celiac sprue). High urinary calcium may suggest renal tubular calcium leak, absorptive hypercalciuria, high sodium diet, or excessive bone resorption due to malignancy, primary hyperparathyroidism, hyperthyroidism or Paget's disease. If celiac disease is suspected, tissue transglutaminase antibodies should be obtained. SPEP, UPEP, and kappa and lambda light chains can be added to the work-up for suspected myeloma.In women without a history of diseases or medications known to adversely affect the skeleton, 32% had disorders of calcium metabolism (hypercalciuria, malabsorption, primary hyperparathyroidism, vitamin D deficiency).(36) Measurement of 24-hour urine calcium, serum calcium, PTH, 25-hydroxy vitamin D and TSH (in those on thyroid replacement) would have been sufficient to diagnose 85% of underlying causes in this group.(36) In another study, except for measurement of TSH, routine laboratory tests were not found to be useful.(37) More specialized tests should also be considered based on clinical suspicion, including serum and urine protein electrophoresis (for myeloma), and a 24-hour urinary free cortisol or overnight dexamethasone suppression test (for suspected Cushing's syndrome).
Markers of bone turnover including various collagen breakdown products may serve to distinguish between high and low turnover bone loss. Serum and urine markers are discussed in detail below under "Monitoring Osteoporosis Therapy."
Bone Mineral Density (BMD) Testing
Dual-energy x-ray absorptiometry (DEXA) is currently the gold standard for measurement of BMD. However, measurement of BMD is not necessary to make the diagnosis of osteoporosis after a fragility fracture. Current T-scores (expressed as the number of standard deviations above or below the normal BMD of young adults) are useful to establish a baseline for purposes of monitoring treatment efficacy and should be performed. Very low Z-scores (expressed as the number of standard deviations above or below the normal BMD for the same aged individuals) may indicate either the failure to obtain adequate peak bone mass during an individual's formative years, or the presence of secondary cause(s) that have contributed to bone loss. In general, the risk of osteoporotic fracture doubles for every drop of one standard deviation in T-score.(38)(39). T-scores are used in postmenopausal women and men >age 50, whereas in young men (age <50) or premenopausal women, Z-scores are typically used to make a diagnosis of osteoporosis.
Lumbar spine and hips are the common sites where DEXA scan is performed. (40) Careful consideration should be given to patients with severe osteoarthritis of spine, as lumbar spine osteophytes can contribute substantially to the lumbar spine BMD measured in the anteroposterior position by DEXA. Also, primary hyperparathyroidism leads to increased bone turnover, low bone mineral density, and increased fracture risk. These effects are, however, preferentially seen in the distal forearm, which is rich in cortical bone. Therefore, distal forearm bone mineral density should be assessed in all patients with primary hyperparathyroidism. (41)
DEXA and similar radiographic techniques only provide information about BMD. High-resolution MRI techniques are being developed, which give the added benefit of more detailed information of the bone's micro-architecture. However, further developments need to be made before this can be clinically used and correlated in practice.
Quantitative CT (QCT) is another method that can be used to determine bone mineral density. (42) QCT is performed on a standard clinical scanner and is highly accurate in determining tissue density within a region of interest. QCT provides a volumetric BMD, in contrast to the areal BMD of the DEXA. Indications for QCT include the same indications as DEXA; however, DEXA is recommended as the first-line screening and follow-up test for bone density, given that radiation exposure is substantially greater when compared to DEXA. If DEXA is not available, QCT may be used as a secondary technique.
Specific cases in which QCT is considered superior to DEXA include: (43)
- Extremes in body height (i.e., very large and very small patients);
- Patients with extensive degenerative disease of the spine;
- A clinical scenario that requires increased sensitivity to small changes in trabecular bone density (parathyroid hormone and glucocorticoid treatment monitoring).
Indications for BMD testing vary among guidelines but those suggested by the National Osteoporosis Foundation (NOF) include: age >65, patients with history of fractures, estrogen-deficient women, hypogonadal men, persons taking long-term corticosteroids, persons with endocrinopathy (such as primary hyperparathyroidism, Cushing's syndrome), persons with significant risk factors regardless of age, assessment of treatment efficacy and persons considering therapy for osteoporosis when BMD will facilitate treatment decisions.(44)(45)
Vertebral fracture assessment (VFA) is a feature of DEXA scanners in which lateral thoracic and lumbar spine images are obtained and screened for fractures. Indications for VFA include: patients with t scores less than –1.0 and one or more of the following: Women age >/=70 years or men age >/=80 years, historical height loss >/=4 cm (>/=1.5 inches), self-reported but undocumented prior vertebral fracture, glucocorticoid therapy equivalent to >/= 5 mg of prednisone or equivalent per day for >/= 3 months. (46)
Treatments and Prevention
The initial evaluation includes referral to an osteoporosis specialist, which can be as part of a fragility fracture pathway, as part of a targeted outreach program to primary care physicians,(47) or as inpatient or outpatient direct consultations to endocrinologists, rheumatologists, geriatricians or other specialists with training in bone disorders. Studies have shown that dedicated interdisciplinary collaboration among orthopedic surgeons, medical specialists in metabolic bone diseases, screening coordinators, nurse-educators and information technologists can play important roles in improving osteoporosis care and therefore potentially help decrease the risk of subsequent fragility fractures.(10)(11)(12)(48)(49)(50). Unfortunately, refracture is still an issue (15).
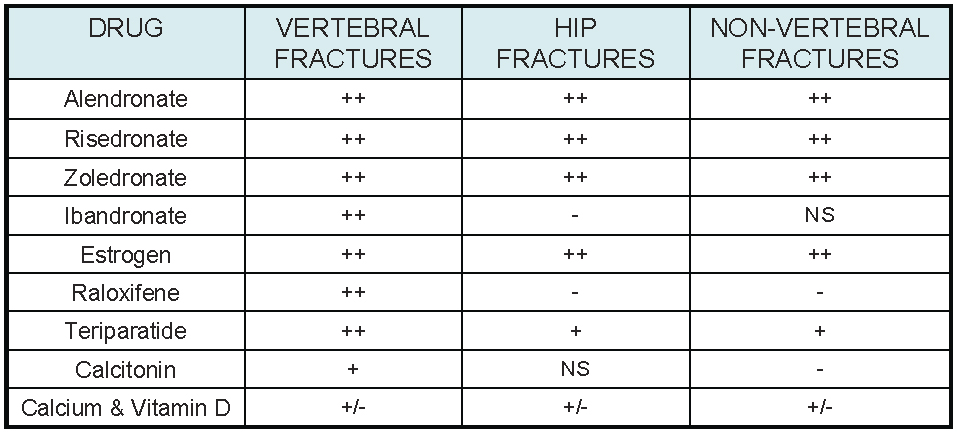
Treatment of osteoporosis and fragility fractures requires a multi-pronged approach: changing modifiable personal risk factors, management of secondary medical causes of bone loss, reduction or elimination of medications that adversely affect mineral homeostasis and bone turnover, improving fall and injury risk, and initiating pharmacological and non-pharmacological interventions that increase bone mass and improve skeletal structural fidelity. Prevention of subsequent fractures requires the early identification and evaluation of patients who sustain an initial fragility fracture, adequate treatment and monitoring of osteoporosis, as well as environmental and activity modifications that address the circumstances of the presenting fracture. The antifracture efficacy of the most frequently used treatments for osteoporosis(51)(52) is shown in Table 3.

Table 3. Antifracture Efficacy Most Frequently Used Osteoporosis Treatments.
Click image for larger view.
From randomized, placebo-controlled trials, in addition to the effects of calcium and vitamin D. ++, strong evidence; +, some evidence; +/-, equivocal evidence; -, weak or no evidence; NS, not studied.

There is a decrease in calcium absorption with age and insufficiency of vitamin D is a common finding (53). There is a positive correlation between 25-hydroxy vitamin D levels and bone mineral density with risk of fractures increased when values are <30 ng/mL (54). Calcium supplementation slightly decreases vertebral fractures (55) but does not lower risk in patients with prior fractures (56). Vitamin D supplementation does reduce fracture risk (57).
For all patients at risk for or who are diagnosed with osteoporosis: ensure adequate dietary calcium (1200 mg daily) and vitamin D levels (treat if serum level <30 ng/mL, and consider maintenance dosing with 2000 IU daily afterwards), decrease alcohol intake (<3 drinks/day) and discourage active cigarette smoking, and encourage 3-4 days a week of weight-bearing exercise. (58-64) Dietary protein is an important factor for continued bone health. (65-70)
In patients with CKD, vitamin D replacement should be performed when 25-hydroxy vitamin D levels are low and target PTH for that CKD stage is exceeded. Inactive forms of vitamin D like ergocalciferol should be used. Active vitamin D is used for CKD stage 3 and 4 when serum 25-hydroxy vitamin D is >30 ng/mL and intact PTH is still above target range. These recommendations are based on the K/DOQI Clinical Practice Guidelines for Bone Metabolism and Disease in Chronic Kidney Disease.
Physical rehabilitation after fracture aims to increase strength and mobility to reduce falls, maintain or build BMD and, in the case of vertebral fracture, decrease kyphotic posture. (71) Prolonged bed rest or severely reduced physical activity cause devastating atrophy of trabecular and cortical bone, as well as decline in numerous body systems. BMD may decline as much as 1-2% per week in both weight-bearing and nonweight-bearing bones, with little or unpredictable recoup after re-initiation of ambulation.(72)(73)(74) To the extent possible, bed rest and reduced physical activity should therefore be minimized in patients with a prior history of fracture.
Educate the patient on mitigating fall risk by modifying home environment: anchor rugs, minimize clutter, remove loose wires, use nonskid mats, all areas should be well-lit, and install handrails in bathrooms, halls, and long stairways. Hip protectors may not be an ideal physical aid to prevent future fracture since long-term adherence is a problem; further, some data suggest that they are clearly ineffective in some populations. (47)(75)
Pharmacologic Treatment
Pharmacologic treatment is indicated if (1) history of fragility fracture, (2) T score <-2.5 or (3) T score between -1.0 and -2.5 with a FRAX score >20% for major osteoporotic fracture or >3% for hip fracture.
Pharmacologic agents can be broadly classified into two categories:
Anti-Resorptive agents which decrease bone resorption:
Anabolic agents which stimulate bone formation:
- Bisphosphonates
- SERMs
- Estrogen
- Denosumab;
- Teriparatide.

Table 4. Characteristics of Drugs to Treat Osteoporosis.
| Category | Drug | Formulation | MOA | Pros | Cons | Comments |
|---|---|---|---|---|---|---|
| Biophosphonates | Alendronate Risedronate Zoledronic acid Ibandronate |
PO PO IV q 12mo PO; IV q 3mo |
Bind to hydroxypatite in bone and reduce activity of bone-resorbing osteoclasts | ------------------ For patients with upper GI issues or malabsorption ------------------ |
Contraindicated: eGFR <30, hypersensitivity, hypocalcemia. Must be able to take fasting in AM w/ full glass of water, remain upright 30-60 min. Counsel on osteonecrosis of jaw, atypical femur fractures. May cause acute-phase reactions in up to 30% of patients |
|
| RANKL inhibitor | Denosumab | SQ q 6mo | Human monoclonal antibody prevents RANKL from binding to RANK (receptor) --> reducing differentiation of precursor cells to mature osteoclasts | For patients with upper GI issues or malabsorption | Correct Ca deficiency, vit D deficiency and secondary hyperparathyroidism to avoid precipitating hypocalcemia | |
| Hormones | Teriparatide Calcitonin |
SQ daily SQ, IM, intranasal |
Recombinant human PTH | Contraindications: patients at risk of osteosarcoma (Paget's disease, open epiphyses, irradiation of skeleton, increased ALKP from skeleton), untreated primary or secondary hyperparathyroidism; SE: nausea, orthostatic hypotension, leg cramps, hypercalcemia, hypercalciuria Contraindication: hypersensitivity. No published studies showing antifracture efficacy |
Can only use for two years. Once stopped, need to follow-up with antiresorptive agent, as effect quickly declines | |
| SERM | Raloxifene | PO daily | Selective estrogen receptor modulator | Also reduces breast cancer risk | Contraindicated: women of childbearing age, previous VTE (increases occurrence threefold), hypersensitivity; SE: menopausal symptoms, leg cramps |
Abbreviations: MOA (mechanism of action); ALKP (alkaline phosphatase); PTH (parathyroid hormone); SE (side effects); VTE (venous thromboembolism.

Bisphosphonates
Primary mechanism of action of bisphosphonates is reduction of bone-resorbing osteoclast activity by binding to hydroxyapatite. Bisphosphonates (alendronate, risedronate, zoledronic acid, and ibrandronate) differ in formulations PO and IV and their relative affinity for bone and their antiresorptive capacity. These differences may translate into clinical consequences such as speeds of onset and duration of effect, relative reduction in bone turnover, uptake in cortical and trabecular bone, and anti-fracture effects (e.g., vertebral vs. nonvertebral). However, except for anti-fracture effects and side effect profiles based on route of administration, there are currently no clear differences.
Oral forms are taken in the morning 30-60 minutes before eating, and with a full glass of water. Upright position should be maintained for at least 30-60 minutes to avoid prolonged contact with the gastrointestinal (GI) mucosa. Bisphosphonates are not recommended in patients with creatinine clearance of less than 30-35mL/min. Potential short-term complications of bisphosphonate use are GI intolerance (heartburn, esophageal irritation, esophagitis, abdominal pain, diarrhea), severe bone, joint and/or muscle pain, ocular inflammation (abnormal or blurred vision, ocular pain, conjunctivitis, uveitis, scleritis) and acute-phase reactions (fever, myalgias, flu-like syndrome).(76)(77)(78) Potential long-term complications include osteonecrosis of the jaw, subtrochanteric and femoral shaft fractures and esophageal cancer.
Osteonecrosis of the jaw (ONJ) results in an area of exposed bone in the maxillofacial region in which there is no healing over eight weeks from the time of discovery. Usual precipitating events include recent tooth extraction or oral surgical procedure, abrasion in trauma-prone location (e.g., mylohyoid ridge in edentulous patients, tori), radiotherapy to the jaw, chemotherapy, and chronic severe periodontitis or other smoldering infection. ONJ may be symptomatic or asymptomatic. It may be infected or non-infected. The incidence of ONJ can be as high as 7% in patients treated with IV bisphosphonates for myeloma or breast cancer, but only 1/100,000 to 1/250,000 treatment-years in patients treated with oral bisphosphonates for osteoporosis.
In acknowledgement of the very low incidence of ONJ when bisphosphonates are used to treat osteoporosis, a 2009 position statement by the American Association of Oral Maxillofacial Surgery concluded: "The current level of evidence does not support a cause and effect relationship between bisphosphonate exposure and osteonecrosis of the jaw."(79) (80) There is no evidence that discontinuing or interrupting bisphosphonate therapy would decrease the risk of ONJ in patients already on bisphosphonates and planning invasive dental procedures, but it can be discussed with the patient.
The link between bisphosphonate use and atypical subtrochanteric and femoral shaft fractures is weak.(81) The incidence of such fractures appears to be very low in comparison with the number of prevented fractures and a causal association has not been established. Recent observations, however, suggest that the risk of atypical fractures increases with the duration of bisphosphonate exposure. The relationship between esophageal cancer and bisphosphonate use is also unclear.
There have been recent studies which suggest an increased risk of new-onset atrial fibrillation with bisphosphonate use. (82) (83) Persistence of oral bisphosphonate therapy is a problem.(84)(85)(86)(87)(88)(89)
Estrogens and Selective Estrogen Receptor Modulators (SERMs)
Estrogen replacement in postmenopausal women, with or without a progestin, reduces vertebral and nonvertebral fractures. The use of hormone and estrogen therapy for this purpose, however, has been limited as a consequence of adverse outcomes reported in the Women's Health Initiative (WHI) study.(90)(91)(92) Therapy with estrogen plus progesterone was associated with an increased risk of deep venous thrombosis (DVT), pulmonary embolism (PE), stroke, myocardial infarction and breast cancer. Estrogen therapy alone was associated with an increase in the risk of DVT, PE and stroke.
Although current guidelines suggest that hormone replacement be used primarily for treatment of menopausal symptoms and for the shortest period of time necessitated for symptom relief, the role of estrogen alone or in combination with a progestin is still far from being adequately explored. Estrogen replacement may currently be considered for osteoporosis treatment after all other alternatives have been exhausted, and when all risks and benefits are fully discussed with the patient.
Selective Estrogen Receptor Modulators (SERMs), such as raloxifene, increase BMD at the spine and hip but only reduce the incidence of vertebral fractures and not hip or other nonvertebral fractures.(93)(94) Among the adverse events that are associated with both estrogen and tamoxifen — including DVT, gallbladder disease, endometrial cancer and cataracts — raloxifene is associated with a higher relative risk for DVT but not other adverse events.(95) Raloxifene may be considered for use in combination with other medications in selected women with or at risk for vertebral fractures. Raloxifene has also been shown to reduce risk of breast cancer in women at high risk (96, 97)
Teriparatide
Teriparatide or PTH(1-34) is the only FDA-approved anabolic agent for bone loss and it confers a reduction of vertebral and nonvertebral fractures in high-risk individuals.(91)(99)(100)(101). It is the only currently available therapeutic agent that increases the formation of new bone tissue and can provide some remediation of the architectural defects in the osteoporotic skeleton. Initial studies on combination or sequential therapy in previously untreated women and men, where PTH analogues were combined with or followed by alendronate treatment, have produced results suggesting no clear benefit to either permutation.(99)(102)(103) Prior long-term alendronate treatment might diminish, but does not eliminate, increase in BMD with use of PTH analogues.(104)(105) It is likely that BMD is lost in individuals who do not take antiresorptive agents after cessation of PTH analogues, and that antiresorptive therapy can maintain or further increase PTH-induced gains.(103)(106)(107)(108)(109)(110). A recent meta-analysis concluded that in patients with osteoporosis, compared with anabolic monotherapy, the concomitant combination therapy of anabolic agents and bisphosphonates significantly improved the BMD at the total hip and femoral neck with a shorter term (6 to 12 months) and produced similar benefits on BMD for the longer term (18 to 24 months). (111)
Teriparatide is currently indicated in men or postmenopausal women who have severe osteoporosis (T-score of -3.5 or below even in the absence of fractures, or T-score of -2.5 or below plus a fragility fracture), have osteoporosis and are unable to tolerate or have contraindications to bisphosphonates, or have fracture and/or loss of BMD in spite of compliance with other osteoporosis therapies.
All PTH analogues should be avoided in those with an elevated risk for osteosarcoma, including individuals with a history of Paget's disease, irradiation or unexplained elevations in alkaline phosphatase. Other contraindications include metastatic bone cancer, multiple myeloma, primary or secondary hyperparathyroidism and hypercalcemia. Treatment with teriparatide is limited to a two-year course because of concerns over the possibility of osteosarcoma; however, no substantiated case of osteosarcoma has been reported to date.(112)(113)(114)
The recommended dose of teriparatide is 20 mcg once a day, administered as a subcutaneous injection into the thigh or abdominal wall. It should be administered initially under circumstances in which the patient can sit or lie down if symptoms of orthostatic hypotension occur.
Denosumab
Denosumab is a human monoclonal antibody that prevents RANKL from binding to RANK (receptor), thus reducing differentiation of precursor cells to mature osteoclasts. It has been approved for the treatment of postmenopausal women with osteoporosis at high risk for fracture, defined as a history of osteoporotic fracture or multiple risk factors for fracture; or patients who have failed or are intolerant to other available osteoporosis therapy. In postmenopausal women with osteoporosis, denosumab reduces the incidence of vertebral, non-vertebral and hip fractures.(115) (116) (117) (118). If denosumab is discontinued, administering an alternative therapy (typically a bisphosphonate) to prevent rapid bone loss is advised.
The recommended dose of denosumab is 60 mg, administered as a subcutaneous injection, every six months. Densoumab, though is usually well tolerated, increases the risk of hypocalcemia, especially in patients with CKD (creatinine clearance <30 mL/min, including patients receiving dialysis) and/or other conditions that predispose to hypocalcemia (malabsorption syndromes, severe vitamin d deficiency). Therefore, these patients should be repleted with vitamin D prior to administration of denosumab and calcium levels should be measured approximately 10 days after denosumab administration.
Monitoring Osteoporosis Therapy
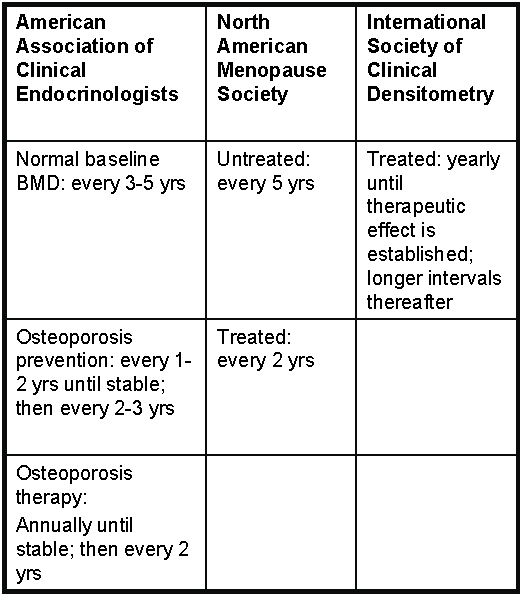
Osteoporosis therapy is generally monitored by regular measurements of BMD because there is a relationship between stability or increase in BMD from antiresorptive therapy and decreased fracture risk. However, increased BMD and decreased fracture risk are not the same for the spine versus non-spine fractures. For example, there are small reductions in the risk of fracture at the spine even without increase in BMD, but not at non-spine locations. In general, reductions in fracture risk with antiresorptive agents are greater than expected from increase in BMD and are not proportional to increase in BMD. Suggested intervals for monitoring by DEXA are based on the observed BMD rate of change with currently available antiresorptive agents and the reproducibility of DEXA BMD testing (Table 5). Changes in BMD >3% are considered real biologic changes but are usually not reached in the spine before one year or in the hip before two years of treatment.

Table 5. Suggested Intervals for Monitoring Changes in Bone Mineral Density By DXA.

Monitoring osteoporosis therapy is sometimes also performed by measurement of bone turnover markers.(119) Serum markers of bone formation are made by osteoblasts (e.g., bone alkaline phosphatase, osteocalcin, C- and N-terminal propeptides of type I collagen). Urine bone resorption markers are breakdown products of type I collagen (pyridinoline [PYR], deoxypyridinoline crosslinks [D-PYR], C- and N-telopeptides of type I collagen [CTx, NTx]).
These markers serve as a dynamic reflection of bone remodeling rates. They do not, however, provide information on existing BMD and cannot confirm the absence or presence of osteoporosis. They are not a substitute for BMD testing but can help to support the diagnosis of high turnover bone loss (e.g., hyperparathyroidism, rheumatoid arthritis). Suppression of bone turnover occurs far more rapidly than detectable changes in BMD and markers reach a nadir within 3-6 months of therapy initiation. The imprecision of measuring markers is far greater than measuring BMD, and cutoff values for the use of markers are uncertain. Desirable levels are considered to be in the premenopausal range, usually at least 20-50% of baseline.
Duration of Treatment
Although bisphosphonates are generally safe and well tolerated, there are some concerns about adverse effects related to long-term use. Their systemic efficacy is related to their binding to bone and a reservoir of bisphosphonates accumulates after years of treatment that is gradually released over months or years and appears to result in a lingering antifracture benefit for some time after therapy is stopped. Therefore, it is possible to consider a 'drug holiday', time off bisphosphonate therapy, and then resuming therapy. The literature supports a therapeutic pause after 3–5 years of bisphosphonate treatment in patients with minor bone deficiencies and no recent fragility fracture (low risk) and in patients with moderate bone deficiencies and/or recent fragility fracture (moderate risk). In these patients, a bone health reevaluation is recommended every 1–3 years. Patients with high fracture risk should be maintained on bisphosphonate therapy without drug holiday. (120) (121)
The FLEX trial showed that if T score <-2.5, then it is more beneficial to treat with alendronate for 10 years compared to five years. However, there was no difference in the first two years after discontinuation of alendronate in the rate of clinical vertebral fractures. (123) (124) This suggests a residual benefit and shows that a 'drug holiday' can be considered. The HORIZON trial showed that after six years of zoledronic acid, there is no difference in clinical fractures when continuing treatment vs placebo over the following three years. (125) And in an extension study of the HORIZON trial after three years of zoledronic acid, there was a significant difference in continuing treatment for an additional three years in morphometric spine fractures but no difference in clinical fractures. (126)
Denosumab should not have a drug holiday because it was shown that BMD decreased back to baseline within two years of discontinuation. (117) Teriparatide use is limited to two years and then should be followed up with an antiresorptive medication because BMD decreases quickly after discontinuation. (109) (103)
Summary
Osteoporosis affects approximately 10% of adults and osteoporotic fractures are associated with increased morbidity and mortality rates. Screen all postmenopausal women >65 years, all men >70 years, and consider screening earlier in patients with non-traumatic fractures, glucocorticoid therapy, low body weight, cigarette smoking, excessive alcohol consumption or known comorbidity causing secondary osteoporosis. The gold standard measurement of bone mineral density is Dual-energy X-ray absorptiometry (DEXA). Using DEXA in conjunction with FRAX scores, treatment is recommended for patients at greater risk for fracture. Treatment is indicated for patient with osteoporosis (T-scores <2.5), or in patients with osteopenia (T-scores between -1 and -2.5) and FRAX 10-year risk of major fracture >20% or hip fracture >3%. Also screen for common causes of secondary osteoporosis with thorough history, physical exam and laboratory tests (CBC, kidney function tests with serum electrolytes, liver tests, albumin, total protein, calcium, PTH, 25-hydroxy vitamin D, phosphorous, magnesium, TSH, serum testosterone). Providers can also consider 24-hour urinary collection for calcium, sodium and creatinine). Lifestyle changes such as adequate dietary protein, calcium and vitamin D intake, decreased alcohol, smoking cessation and increased weight-bearing exercises are advised. Pharmacologic treatment is dependent on patient comorbidities, severity and location of osteoporosis and side effect profiles of medications. Continue to monitor BMD using DEXA scans during pharmacologic treatment according to guidelines.