Open Angle Glaucoma
Course Authors
Enoch B. Kassa, M.D., and Yang Sun, M.D., Ph.D.
Dr. Kassa Is Resident Physician, Ophthalmology, Eugene and Marilyn Glick Eye Institute, Indiana University School of Medicine, Indianapolis, IN, and Dr. Sun is Associate Professor of Ophthalmology, Stanford University School of Medicine, Palo Alto, CA.
Within the past 12 months, Drs. Kassa and Sun have no conflicts of interest relevant to this activity.
Albert Einstein College of Medicine, CCME staff, and interMDnet staff have nothing to disclose relevant to this activity.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Discuss the epidemiology and risk factors of open-angle glaucoma;
Describe the process of diagnosing open angle glaucoma;
Discuss the management of open angle glaucoma, the outcomes and new therapies.
Glaucoma is a group of eye diseases that cause damage to the optic nerve with specific patterns of visual field loss that can lead to blindness. Glaucoma subtypes include open angle glaucoma (OAG) and angle-closure glaucoma (ACG). OAG is further characterized into primary open angle glaucoma (POAG), normal tension glaucoma (NTG), and OAG caused by other conditions (i.e., secondary OAG).
The most common type of glaucoma is primary open angle glaucoma (POAG). OAG affects 2.22 million Americans and POAG is responsible for over 70% of adult glaucoma diagnoses.(1) The majority of patients with OAG have the following characteristics: an increase in intraocular pressure (IOP), an open angle between the cornea and the iris, and a slow and asymptomatic progression of the disease. These elements of OAG distinguish it from other types of glaucoma such as angle-closure glaucoma (ACG), which, as its name implies, has a closed angle with rapid progression of vision loss, usually with pain.
An increased IOP is the major risk factor for glaucomatous optic nerve damage and the resulting vision loss in OAG. The pathophysiology behind this process of nerve damage is unclear; however, the mechanism behind the increase in IOP is due to the dysregulation of production of aqueous humor from the ciliary body and a decrease in the outflow of aqueous humor by the trabecular meshwork. Current glaucoma research focuses on investigating the mechanism behind optic nerve damage and the various processes that may increase IOP.
The loss of vision in POAG is typically slow and may take many years prior to diagnosis.(2) Glaucomatous vision loss is irreversible and can dramatically decrease the quality of life for the patient. Thus the early diagnosis and management of OAG is vital to the prevention of blindness.
This Cyberounds® will review the screening, diagnosing and management guidelines that help physicians treat OAG. Previous research in these fields has led to a number of medical and surgical treatments for glaucoma. It is, however, important to note that these treatments only slow the disease process and that, currently, no definitive cure for glaucoma is available. The primary goal of glaucoma therapy is, therefore, to slow the progression of vision loss in order to minimize the functional impairment for patients.
Epidemiology
Glaucomatous vision loss is irreversible.
Glaucoma is the second leading cause of blindness in the world with 60 million people afflicted by the disease.(3) Three quarters of the 60 million people with glaucoma have OAG. OAG is also responsible for over half of the 8.4 million people who have lost their vision. The prevalence of glaucoma is predicted to increase to 80 million and the prevalence of blindness due to glaucoma is predicted to increase to 11.2 million by 2020. Women, who account for 55% of OAG, are more affected than men by glaucoma.(3) In the United States, African Americans have the highest prevalence (up to 6 times) of OAG compared to other ethnic populations.(4) Glaucoma affects people of African origin at a younger age and the disease process is more rapid than in Caucasian patients.(5)
People in developing countries are at increased risk of blindness from OAG compared to developed countries. This is caused by a lack of adequate healthcare, expense of medications, lack of disease education, lack of visual improvement with medications and discouraging side effects.(6) Unlike cataracts, where treatments can result in a dramatic improvement of vision, glaucoma treatments only slow the progression of disease and these patients may not notice improvement with vision and may stop using the medicines. A study in Brazil, which is a developing country, showed a 21.5% rate of non-adherence to treatment regimen for glaucoma.(6) Thus, compliance with glaucoma treatment is a huge issue and needs to be addressed in order to improve outcomes for glaucoma.
As one of the main causes of blindness in the world, OAG needs to be diagnosed and managed to prevent dramatic decreases in quality of life and even death in regions where vision is vital for survival. In developing countries where access to health care is limited, glaucoma becomes an increasingly important public health challenge that requires first identifying and educating those afflicted with glaucoma.
Pathophysiology
The mechanisms for optic nerve degeneration in OAG have not been well established. The longstanding theory is that increased IOP causes optic nerve damage and vision loss.(7) However, a closer look at the OAG population points to a more complicated mechanism. It has been discovered that there are patients with normal IOP who develop glaucoma, known as normal tension glaucoma (NTG). And not all patients with elevated IOP develop glaucoma. These two patient populations suggest that increased IOP is not the only feature in this disease process, albeit it remains a very important risk factor since the majority of patients with OAG have increased IOP.(8) Patients with NTG still benefit from therapy that maintains or lowers their IOP, suggesting that IOP plays a significant role.(9)(10)
This unclear picture of the pathophysiology behind OAG has sparked much glaucoma research focusing on new pathways for optic nerve damage. But the mechanism of increased IOP remains important because it is a major risk factor for OAG and all treatments hinge on regulating ocular pressure.
IOP is regulated by aqueous humor, which is produced in the ciliary body and released into the posterior chamber. It then travels through the pupil to the anterior chamber where it is funneled and drained into Schlemm's canal by the trabecular meshwork at the angle of the iris and cornea. From the Schlemm's canal, the aqueous humor is reabsorbed by the surrounding venules. A decrease in the absorption of aqueous humor by the trabecular meshwork will cause an increase in IOP as the eye is unable to drain excess fluid.
Secondary causes of OAG have been identified and include neovascularization, pigment dispersion, pseudoexfoliation syndrome, infection and trauma. These processes affect the outflow pathway — the ability of the trabecular meshwork to drain aqueous humor.
Angle-closure glaucoma occurs when the iris blocks the angle and prevents the trabecular meshwork from draining the aqueous humor, which leads to an acute increase in IOP. If not treated immediately, the increased IOP produces rapid vision loss. OAG is a more chronic process that occurs with an unobstructed angle.
Glaucoma is the second leading cause of blindness in the world.
Presentation and Diagnosis
OAG patients usually have no symptoms upon presentation because the disease is slowly progressive and loss of central vision does not happen until years into its course. Peripheral vision loss usually precedes central vision loss, but, typically, it is not noticed by the patient. The lifetime risk for blindness in one eye is approximately 40%, and for both eyes it is approximately 15%.(11) The lack of symptoms for OAG and its insidious progression require ophthalmologists to have a high index of suspicion in patients who are at high risk for developing this disease.

Table 1. Risk Factors for OAG.
| African heritage |
| Increased age |
| High refractive error |
| Decreased central corneal thickness |
| Increased optic disc diameter |
| Increased IOP |
| Cardiovascular disease |
| Low physical activity(12)(19)(20)(21)(22)(23) |

The diagnosis of POAG is made upon routine eye exam and with the aid of additional OAG tests described below. Patients with either visual field defects or optic neuropathy on fundus exam, usually in the setting of elevated IOP, qualify for the diagnosis of POAG. Other diagnostic criteria include an adult onset, lack of secondary causes for either the visual field defects or increased IOP, and open iridocorneal angles.(12)
The diagnosis of OAG due to secondary causes is made with careful history taking and a comprehensive eye exam. Any past steroid use or trauma, signs of uveitis, presence of neovascularization or pigment cells point towards OAG. The presentation of OAG, but with no elevation in IOP, suggests a diagnosis of NTG.
Screening for OAG has not been well established. While IOP is a major risk factor and monitoring it aids the management of glaucoma, using IOP as a screening tool by itself has been unreliable because of inadequate sensitivity.(13) This insensitivity results from the fact that a significant minority of patients with OAG have no elevations in IOP and a screening test using IOP would miss these patients. Also, not all patients with increased IOP develop OAG. Therefore, it has been suggested that screening with tonometry alone is subpar.(14) It is now recommended that all patients over the age of 40 receive a comprehensive eye exam, which includes measuring ocular pressure, to effectively screen for OAG. Those with increased risk factors may benefit from an earlier eye exam to screen for OAG.(15)
The ophthalmologist will perform a visual field test and a dilated optic nerve examination as part of the comprehensive evaluation.(16) Patients who have OAG for several years may show signs of optic nerve damage and visual field loss on examination. On dilated optic nerve exam, the patient may have optic disk abnormalities such as thinning, cupping or notching of vessels as they exit the disk. See Figure 1 for an example of optic disk findings in OAG.
Visual field perimetry is the gold standard test for detecting optic nerve functional changes.

Figure 1. Optic Nerves In Glaucoma.
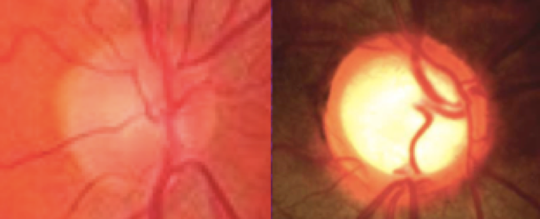
Optic disk cupping in a healthy patient (left) compared to a patient with OAG (right).

Visual field perimetry is the gold standard test for detecting optic nerve functional changes. This test has the patient look into the field machine and their response to a small flash of light is recorded and their visual field mapped. See Figure 2 for examples of visual field findings in patients with OAG.

Figure 2. Visual Field Defect In Glaucoma

Results of white-on-white perimetry in a patient with visual field loss due to OAG.

Patients in the early stages of OAG may have no findings on these exams and visual field defects only manifest after a significant loss (40%) of one's retinal ganglion cells.(17) Since increased intraocular is the strongest predictor of future development of glaucoma, the ophthalmologist will still treat these patients by lowering their pressures in an attempt to prevent development of the disease.(18) Management for patients with increased IOP, but with no signs of OAG on examination, is discussed in the management section below.
Other testing methods that are utilized to aid in the diagnosis of glaucoma include pachymetry and gonioscopy. Pachymetry measures the thickness of the cornea; a thin cornea of less than the average 540 um may predispose patients to OAG. Gonioscopy utilizes a special lens to view the angle between the iris and cornea, allowing the ophthalmologist to view the trabecular meshwork. Slit lamp exam combined with gonioscopy can help rule out ACG and secondary causes of glaucoma such as neovascular glaucoma, pigment dispersion syndrome and pseudoexfoliation syndrome. The diagnosis of POAG cannot be made without first ruling out these other pathologies.(12)
Several new imaging modalities may also be helpful and they are discussed in more detail in the management section below. These imaging devices include Optical Coherence Tomography (OCT) and Heidelberg Retinal Tomograph (HRT), which help the ophthalmologist look for subclinical signs of optic nerve damage. These modalities are being actively studied for potential use in screening.
Management
First line therapy for lowering increased IOP is medical therapy.
Lowering the IOP in patients with OAG significantly decreases the progression of the disease as measured by visual field loss and optic nerve cupping.(24) IOP remains the only approved therapy for the treatment of OAG.(25) Early therapy is paramount in order to prevent blindness.(9) Lower IOP in patients with normal tension OAG also decreases visual loss.(10) Therefore, treatment for OAG involves decreasing IOP by manipulating one or both of the disease processes that raise IOP (described above) increased production of aqueous humor from the ciliary body and decreased elimination of aqueous humor by the trabecular meshwork or the uveoscleral outflow.
Patients who do not have the diagnosis of OAG because of negative fundus examinations or normal visual field tests, but have increased IOP (also known as ocular hypertension), are at a higher risk of developing the disease in the future or may even be in the early stages of the disease.(19) The ophthalmologist will treat their IOP with the goal of preventing the onset of OAG.
The objective for all treatments is to lower IOP to prevent further optic nerve damage. There is no set IOP target and the physician must decide the optical pressure for each patient. The physician will do a dilated fundus exam, optic cup measurement, visual field testing and retinal nerve fiber layer (RNFL) imaging such as OCT to characterize the progression of OAG.(15) Additional imaging modalities are described later in this section.
First line therapy for lowering increased IOP is medical therapy. If medical therapy fails to lower IOP, the next line of treatment is laser surgery aimed at the trabecular meshwork. If the patient's disease is also refractory to laser therapy, then surgery is indicated.(26) If all of these treatments fail, the last resort is another category of therapies called cyclodestructive, which targets the ciliary body where the production of aqueous humor occurs.(12)
OAG Treatment
Medical Therapy
Medical therapy in the form of eye drops is first line therapy because it is non-invasive and effective. Table 2 lists the various medical therapies used and their mechanism of action. It is important to consider the side effects of these medications since these drugs in the form of eyedrops are drained by the lacrimal duct system and can be absorbed directly into the blood stream by the nasal epithelium, which bypasses liver metabolism. Thus, the systemic effects of these drugs can be significant. For example, when using beta blockers, the ophthalmologist must take a careful medical history from the patient and search for a history of COPD, asthma or heart disease, as beta blockers can exacerbate these conditions. If the patient is already on beta blockers for another condition, beta blocker eye drops may not be as effective.

Table 2. Medical Therapies.
| Beta blockers lower IOP by decreasing aqueous humor production in the ciliary body. |
| Alpha 2 adrenergic agonists work similar to beta blockers by decreasing aqueous humor production but also increase uveoscleral outflow. |
| Prostaglandins work by increasing uveoscleral outflow of aqueous humor. |
| Carbonic anhydrase inhibitors decrease the production of aqueous humor in the ciliary body. |
| Parasympathomemetics contract the ciliary muscle and open up the trabecular meshwork, which improves outflow.(12) |

The most commonly used first line agent is prostaglandins — they have been shown to be more effective compared to other medications and do not have beta blockers' particular side effects.(12) Prostaglandin side effects include increasing the length of eyelashes and darkening of the iris color.[12] Combining multiple types of eye drops can be done to increase efficacy if the patient has a minimal response to monotherapy.(27)(28)
Cannabinoids have been shown to reduce IOP only temporarily. Long-term management with cannabinoids has not been shown to be effective because IOP eventually becomes unresponsive to the therapeutic effects of cannabinoids and returns to elevated levels.(29)
Laser Surgery
If medical therapy fails, laser surgery is the next line of treatment. The three types of laser surgery are argon laser trabeculosplasty (ALT), selective laser trabeculoplasty (SLT) and micropulse laser trabeculosplasty (MLT). By aiming these lasers specifically at the trabecular meshwork, the lasers cause the channels to become more open and improve outflow of aqueous humor. Laser therapies can be repeated as they cause little to no harm to the patient. A recent study has shown that laser surgery is just as effective as medical therapy and there are indications that laser therapy should be first line.(30) In patients that adhere to medication regimens poorly, laser therapy may provide better outcomes. Laser therapy may also be combined with medications to augment therapy.
ALT was the first laser treatment for glaucoma and it uses a thermal beam to cause scarring of the trabecular meshwork that induces the healing response at that site. This results in the opening of the trabecular meshwork via remodeling and increased outflow of the eye.(12)
SLT uses a non-thermal beam that works like ALT and has similar efficacy. The target area is larger but the energy is lower, resulting in less damage. The potential benefit of this procedure compared to ALT is the hypothetical repeatability of this treatment for the patient. Studies are currently underway to demonstrate that SLT is more effective than ALT upon repeat laser treatment.(31)
MLT delivers energy to the trabecular meshwork in short bursts instead of a continuous bundle of energy. In theory, this will decrease the amount of damage to the trabecular meshwork, resulting in increased repeatability of the procedure and its tolerability by the patient.(32)
Lowering the IOP in patients with OAG significantly decreases the progression of the disease.
Surgery
Surgery is utilized after both medical and laser therapies have failed, but it is accompanied by the inherent risks of surgery and increased likelihood of failure compared to other therapies.(12) The ophthalmologist has a wide range of surgeries to choose from including minimally invasive glaucoma surgery (MIGS) and more invasive traditional glaucoma filtration surgeries. The choice of surgery depends on the disease severity and the patient's tolerance to surgery. For example, patients with mildly elevated IOP and minimal optic nerve damage may be suitable candidates for MIGS. MIGS are new procedures that are continually being investigated and compared to traditional surgical procedures at this time. Theoretically, they cause fewer surgical complications but they may have a higher failure rate.(33)
One type of MIGS is called a trabectome, a procedure that removes part of the clogged trabecular meshwork at the anterior segment angle so aqueous humor can be more easily absorbed by Schlemm's canal. The procedure utilizes a tool called the Trabectome to electrically ablate a small strip of trabecular tissue.(34)
Canaloplasty is another minimially invasive surgical technique that dilates Schlemm's canal to improve outflow. A microcatheter is threaded through the entire 360 degrees of the canal. A suture is tied to the distal end of the catheter and the catheter is pulled backwards to thread the suture through the canal. The two ends of the suture are tied with tension to maintain dilation of Schlemm's canal.(35)
The most commonly performed surgical procedure for glaucoma in patients who have failed medical and laser therapy is the trabeculectomy.[12] It is also utilized in patients who present with advanced glaucoma. Trabeculectomy involves the formation of an ostium between the anterior chamber and the subconjuctival space through a scleral flap. The aqueous humor flows into the space and forms a filtering bleb, thus providing an alternative pathway for aqueous humor to flow out of the anterior chamber. Due to scarring, this procedure has a failure rate as high as 39% at 5 years.(36) Adjunctive antimetabolites are able to slow down the scarring and allow the trabeculectomy to remain patent but may increase the risk of other side effects such as cataract formation.(37)
The Ex-press mini shunt is a metal device that is inserted into the anterior chamber from underneath a scleral flap and works in combination with a trabeculectomy. It was developed to improve the trabeculectomy (with its high failure rate) and provides a more stable platform for drainage. The Ex-press mini shunt has a barb at the front to prevent dislodgment and an external plate in the back to prevent protrusion into the anterior chamber. This technique is popular among ophthalmologists due to its ease of use, smaller incision, quicker recovery and lower complication rates.(38) Ongoing studies will determine whether this procedure is more effective than the standard trabeculectomy.
For more severe cases of glaucoma that are refractory to medical and laser therapy, and with an increased chance that a filtration bleb will fail due to previous filtration bleb failure, neovascular glaucoma or corneal transplant, a tube-shunt may be placed to relieve IOP. The flexible plastic tube-shunt can be surgically inserted into the anterior chamber and connected to a plate that sits on the surface of the eye behind the conjunctiva to allow for outflow. Like the trabeculectomy, the shunt also creates an alternate pathway between the anterior chamber and the subconjunctival space, allowing aqueous humor to escape and be absorbed by scleral veins. Some devices have valves to prevent back flow and to prevent hypotension in the eye by regulating rate of outflow. The tube-shunt has a lower failure rate than the trabeculectomy.(39)
Cyclodestructive Procedures
When OAG is refractory to all the treatments listed above or have contraindications to those treatments, cyclophotocoagulation can be utilized to decrease IOP. Cyclophotocoagulation uses a laser to ablate at least 270 degrees of the ciliary body to limit aqueous humor production in order to decrease IOP. This procedure involves a limbal or pars plana incision to allow the laser endoscope to access and view the ciliary body. This procedure has a 90% success rate but carries side effects such as hypotony.(12)(26)
Monitoring/Follow Up
For optimal management of OAG, patients should be seen regularly and have their IOP test, an optic disc exam, a visual field test and additional imaging as needed. The ophthalmologist will continue to follow and treat the increased IOP. Since there is no definitive cure, patients will have to be seen for their entire lifetime. Less frequent testing is needed once optimal treatment and pressures have been reached. Ophthalmologist will usually see OAG patients once a month until progression has halted and afterwards twice yearly. The physician should also be aware of any patient non-compliance issues and should choose therapy accordingly.
Imaging
Various new imaging technologies track the progression of the disease and help make treatment decisions.
Heidelberg retinal topography (HRT), optical coherence tomography (OCT) and scanning laser polarimetry (SLP) are relatively new and non-invasive imaging modalities that improve the treatment and management of OAG.
HRT uses the reflection of light off tissues to create a three-dimensional image of the optic disk. The technology's software then measures the different parameters of the eye to screen for the disease and to track any structural changes of the optic nerve. Different algorithms are being tested and have continued to improve the effectiveness of this imaging modality.(40)
Both OCT and SLP measure the thickness of the retinal nerve fiber layer (RNFL). OCT uses low-coherence light in a manner similar to ultrasound to create a cross-sectional image of the RNFL. SLP measures the RNFL thickness using a confocal laser that allows the instrument to calibrate the birefringence of optic nerve cell axons. It's important to measure the RNFL because it can be extrapolated to vision loss and lead to early detection of optic nerve damage, and thus earlier diagnosis of OAG. As mentioned above, a significant percentage of the optic nerve is already damaged before vision loss is detected on white-on-white perimetry.(41)(42)(43)
At this time, studies have shown that OCT is one of the most accurate tests for monitoring glaucoma compared to HRT or SLP.(44) OCT is becoming widely adopted by ophthalmologists to assist in the management of this disease.
New Therapies on the Horizon
Latanoprostene bunod is a nitrous oxide-donating prostaglandin that increases uveoscleral outflow and increases trabecular outflow. Phase 2 trials show that this drug decreases IOP better than latanoprost with a similar degree of side effects.(45)
A new class of drugs — rho kinase inhibitors — work by shrinking the cell cytoskeletons of the trabecular meshwork and adjacent area, allowing fluid to exit. These drugs are being tested alone and in combination with other medical therapies to treat glaucoma. They have been shown to reduce IOP in preliminary human studies and will potentially be added to the arsenal of drugs used to treat OAG.(46)(47)