Vitamin D in Health and Disease
Course Authors
Malcolm D. Kearns, M.D., and Vin Tangpricha, M.D., Ph.D.
Malcolm D. Kearns, M.D., is Medical Resident, and Vin Tangpricha, M.D., Ph.D., is Associate Professor of Medicine, Division of Endocrinology, Metabolism and Lipids, Department of Medicine, Emory University School of Medicine, Atlanta, Georgia.
Within the past 12 months, both Drs. Kearns and Tangpricha report no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff, and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Describe the physical properties of vitamin D and its roles in the body
Evaluate the meaning, measurement and epidemiological data of vitamin D sufficiency
List the skeletal and non-skeletal pathologies related to vitamin D deficiency
Apply the methods of treatment and prevention for vitamin D deficiency.
In the early twentieth century, McCollum and colleagues discovered that a component of cod liver oil could cure rickets in dogs.(1)(2) Following the pattern of naming vitamins A through C, this factor became known as vitamin D, the fourth vitamin discovered. Since its initial use in the treatment of bone disease, the potential therapeutic applications of vitamin D have broadened, as vitamin D receptors have been identified in numerous tissues and immune cells. Considered a miracle drug by some and a fad by others, vitamin D has received increasing attention over the past decades for its potential role in cardiovascular disease, pregnancy, infection and malignancy, among other diseases.
With vitamin D deficiency present in nearly half of the healthy population of developed countries,(3) research has focused on treating and preventing vitamin D deficiency through increased sun exposure, food fortification and vitamin D supplementation. Results have, however, been variable, making it difficult to draw definitive conclusions about the many potential roles of vitamin D in the body.
In this Cyberounds® we will address: (1) the properties of vitamin D, (2) the meaning, measurement and epidemiological data on vitamin D sufficiency, (3) pathologies associated with vitamin D deficiency and (4) methods of providing treatment and prevention for vitamin D deficiency.
What Do We Mean By Vitamin D?
The term vitamin D refers to a group of fat-soluble secosteroids (a steroid in which a bond in one of its rings is broken). In humans, two major forms of vitamin D exist: vitamin D3, or cholecalciferol, and vitamin D2, or ergocalciferol.(4) Though some vitamin D2 and D3 can be acquired through diet,(4) the synthesis of vitamin D3 in the skin with sunlight exposure remains the key determinant of the body's vitamin D status.(5) Ultraviolet-B (UVB) radiation converts 7-dehydrocholesterol to previtamin D in the skin before rapidly isomerizing previtamin D to vitamin D3.
Vitamin D2 and D3 are converted into the active form, 1, 25-dihydroxyvitamin D 1,25(OH)2D, or calcitriol) by two hydroxylation reactions (Figure 1). The first reaction occurs via the enzyme 25-hydroxylase in the liver,(6) forming 25-hydroxyvitamin D 25(OH)D. 25(OH)D is the circulating form of vitamin D(7) in part because of its two- to three-week half-life.(8)(9) This relatively long half-life is due to vitamin D binding protein (DBP), which binds 85-90% of 25(OH)D.(10) DBP slows the degradation of 25(OH)D and increases its reabsorption when filtered by the kidney.(11)(12)
Synthesis of vitamin D3 in the skin with sunlight exposure remains the key determinant of the body's vitamin D status.
Vitamin D deficiency is present in nearly half of the healthy population of developed countries.

Figure 1. The Synthesis and Activation of Vitamin D.

Vitamin D2 and D3 are consumed in the diet, while only vitamin D3 is synthesized in the skin by the conversion of 7-dehydrocholesterol to previtamin D with exposure to UVB radiation. Both vitamin D2 and D3 undergo two hydroxylation reactions in order to form the active form of vitamin D, 1,25-dihydroxyvitamin D, or 1,25(OH)2D. The first reaction occurs by the enzyme 25-hydroxylase in the liver, while the second occurs most prominently in the kidneys through the enzyme 1-alpha hydroxylase or CYP27B1. 1,25 dihydroxyvitamin D is converted to an inactive form, 1,24,25(OH)3D, before being excreted in the bile.

The serum concentration of 25(OH)D has traditionally been measured to determine vitamin D status, though only approximately 15% of the total 25(OH)D is bioavailable and freely accessible to cells.(13) New assays that measure free vitamin D may prove to be a more accurate in determining vitamin D sufficiency (see: Future directions below).
A second hydroxylation occurs via the P450 enzyme 1 alpha-hydroxylase, or CYP27B1, and converts 25(OH)D to 1,25(OH)2D in the proximal tubules of the kidneys(14) and other organs targeted by vitamin D.(15) The relatively short-lived 1,25(OH)2D (half-life of 10-20 hours)(16)(17) enters target cells and acts through the vitamin D receptor (VDR),(18), which has been identified in numerous tissues throughout the body.(20) The VDR is a nuclear receptor that joins with the retinoic acid X receptor (RXR) before entering the nucleus, binding deoxyribonucleic acid and increasing transcription of target genes.(21)(22) Over 800 human genes contain a vitamin D response element.(23)
Once formed, 1,25(OH)2D induces its own deactivation by increasing synthesis of 24-hydroxylase, or CYP24A1.(24) CYP24A1 is located in the kidney and other tissues targeted by vitamin D(25)(26) and changes 25(OH)D into 24,25(OH)2D and 1,25(OH)2D into 1,24,25(OH)3D, or calcitroic acid, which is excreted in bile.
What Is Vitamin D Deficiency?
Vitamin D status is determined by measuring the body's total serum 25(OH)D concentration, which includes 25(OH)D formed from both vitamin D2and D3. 25(OH)D is a good measure of vitamin D sufficiency because it is the predominate circulating form of vitamin D and believed to mediate vitamin D toxicity when in excess.(27)
Interpretation of vitamin D sufficiency is somewhat controversial. The Endocrine Society considers serum 25(OH)D concentrations >30 ng/mL to be sufficient and concentrations 21-29 ng/mL and <20 ng/mL to be insufficient and deficient, respectively.(28)(29) This recommendation is based on the concentrations of 25(OH)D determined to optimize biochemical markers regulated by vitamin D. For example, intestinal calcium absorption is optimized at serum 25(OH)D concentrations between 30-32 ng/mL(30) and parathyroid hormone (PTH) concentration (a marker of bone turnover inversely correlated with vitamin D) begins to rise at 25(OH)D concentrations <31 ng/mL.(31) However, the Institute of Medicine (IOM) found no consistent benefit associated with 25(OH)D concentrations >20 ng/mL.(32)(33)
Who Is Vitamin D Deficient?
With nearly half of the healthy population,(3) and even more of many diseased populations, vitamin D deficient, The Endocrine Society recommends screening only individuals who are at high risk for vitamin D deficiency.(28) It is important to understand the potential mechanisms of vitamin D deficiency (Table 1) in order to be able to identify at-risk populations and determine who to screen.

Table 1. What Causes Vitamin D Deficiency?
| Category | Cause | Mechanism |
|---|---|---|
| Decreased vitamin D synthesis | Inadequate sun exposure Seasonal changes in UVB radiation Increased skin pigmentation Age-related skin changes |
Those with limited sun exposure (e.g., indoor occupations,(34) individuals in the hospital(35) or other institutions)(36) do not synthesize adequate vitamin D. Above 35° latitude, seasonal changes in the zenith angle of the earth to the sun lead to changes in the amount of UVB radiation penetrating the Earth's surface.(37) Vitamin D levels are typically lowest following the winter and highest following the summer. Individuals with darker skin require more UVB radiation to produce the same amount of vitamin D as a lighter-skinned individuals(38)(39) since melanin absorbs UVB radiation needed for vitamin D synthesis.(40)(41) Older individuals have less 7-dehydrocholesterol in their skin(42) and thus produce less vitamin D by cutaneous sunlight exposure.(43) |
| Decreased absorption of dietary vitamin D | Malabsorptive diseases of the gastrointestinal tract Low vitamin D intake in breast milk Medication effects |
Vitamin D is absorbed in the intestines along with fat. Thus, malabsorptive diseases, such as cystic fibrosis (CF), inflammatory bowel disease (IBD), short bowel syndrome (SBS), decrease the absorption of vitamin D along with that of other fat soluble vitamins.(40)(44)(45) Breast milk is very low in vitamin D.(46) It is recommended that infants receive vitamin D supplementation until they can consume adequate fortified formula or whole milk.(47) Certain medications, such as the cholesterol-lowering drug cholestyramine and the weight loss drug Orlistat, reduce intestinal fat absorption(48)(49) and thus can cause vitamin D deficiency by malabsorption. |
| Inability to activate vitamin D | Damage to organs that activate vitamin D | Individuals with kidney(19) and liver(52) disease are often unable to activate vitamin D and may experience symptoms consistent with hypovitaminosis D. |
| Increased sequestration of vitamin D | Obesity (BMI >30 kg/m2) | Vitamin D is fat soluble. Individuals who are obese require more vitamin D to achieve an equivalent increase in 25(OH)D as normal weight individuals,(40) since a greater amount of vitamin D is sequestered in subcutaneous fat stores.(51) |
| Increased loss of vitamin D | Nephrotic syndrome | Individuals with proteinuria lose increased levels of proteins, such as DBP, which reduces filtered 25(OH)D that is reabsorbed in the kidney.(12)(52) |
| Increased catabolism of vitamin D | Medication effects | Certain medications, such as glucocorticoids(53) and anti-convulsants,(54) increase the catabolism of vitamin D. |
Abbreviations: 25(OH)D, 25-hydroxyvitamin D; BMI, Body Mass Index; CF, Cystic Fibrosis; DBP, vitamin D binding protein; IBD, inflammatory bowel disease; sBs, short bowel syndrome; UVB, Ultraviolet B;
The Endocrine Society recommends screening for vitamin D deficiency (measuring serum 25(OH)D concentration) only in high-risk populations.(28) Recognizing the causes of vitamin D deficiency is essential to determining who is at-risk for vitamin D deficiency and thus who to screen.

The major cause of vitamin D deficiency is the inadequate synthesis of vitamin D in the skin. Reduced sun exposure, such as from minimal time outdoors or over-use of sun protection, is one cause of sub-optimal vitamin D synthesis(55)(56)(57)(58) affecting both older patients in nursing homes (36) or hospitals(35) and younger individuals who work exclusively indoors. A recent study showed nearly all young, healthy adults in a cohort who spend <10 hours/week outdoors to be vitamin D deficient or insufficient at baseline.(34) Seasonal variations in sun exposure also contribute to vitamin D insufficiency;(59)(60) during winter, changes in the zenith angle of the Earth to the sun cause less UVB radiation to reach the Earth's surface(37) and contribute to the well-documented decline in vitamin D status following winter months.(61)(62)(63)(64)(65)
Intrinsic characteristics of the skin, such as aging and skin pigmentation, can also reduce vitamin D synthesis and lead to insufficiency.(40)(41)(43) While older adults synthesize less cutaneous vitamin D3 (a 70-year-old's skin has 25% of the 7-dehydrocholesterol of a 20-year-old's skin(42)), people with darker skin also experience reduced cutaneous vitamin D synthesis(40) and a higher prevalence of vitamin D insufficiency compared to lighter-skinned individuals.(38)(39)
Data from the third National Health and Nutrition Examination Survey (NHANES III) show that 53-76% of non-Hispanic blacks were vitamin D deficient in the winter, while only 8-33% of non-Hispanic whites were vitamin D deficient.(66) A study in pregnant women reported similar findings; 47% of Asian women, 64% of Middle Eastern women and 58% of African American women were vitamin D deficient compared to 13% of Caucasian women.(67) This phenomenon is likely because increased UVB radiation is required to account for the radiation absorbed by melanin in darker-skinned individuals;(40)(41) non-Hispanic black subjects required six times more UVB radiation than non-Hispanic white subjects to produce similar increases in serum 25(OH)D concentration in one study.(68)
The low level of vitamin D in breast milk (<25-78 IU/L depending on the mothers vitamin D status)(47) makes infants susceptible to vitamin D deficiency. The American Academy of Pediatrics (AAP) recommends that all infants receive 400 IU/day of vitamin D until they consume >1000 mL/day of vitamin D-fortified formula or whole milk.(47) Vitamin D deficiency often continues into childhood; data from NHANES found that nearly 1 in 10 children have serum 25(OH)D concentrations <16 ng/mL.(69) Children aged 14-18 years, who are female, nonwhite and obese were at the greatest risk for vitamin D insufficiency.(69) Various other mechanisms known to cause vitamin D deficiency are linked to disease processes. For example, since vitamin D is absorbed with dietary fat,(70) diseases with poor fat absorption (e.g., celiac disease, short bowel syndrome, liver disease, cystic fibrosis (CF) and inflammatory bowel disease) can lead to vitamin D deficiency.(40)(44)(45) Vitamin D deficiency is also prevalent in populations with damage to the organs that activate vitamin D, such as patients with end stage liver(50) or chronic kidney disease (CKD).(19) Kidney disease with proteinuria can also result in vitamin D deficiency from the loss of vitamin D binding protein (DBP) in urine and thus reduced reabsorption of filtered 25(OH)D.(12)(52)
Low level of vitamin D in breast milk makes infants susceptible to vitamin D deficiency.
Physiologic Functions Of Vitamin D And Associated Pathologies
Vitamin D sufficiency is necessary for optimal bone health, since calcitriol increases intestinal calcium and phosphorous absorption(9)(71) to the concentrations necessary to adequately mineralize the skeleton.(72) The gut's ability to absorb calcium is greatly dependent on vitamin D concentration. Optimal calcium absorption (30-40% of dietary calcium)(73) occurs at 25(OH)D concentrations between 30-32 ng/mL,(30) while at lower vitamin D states only 10-15% of dietary calcium is absorbed.
Vitamin D also plays a role in the maturation of osteoclasts, which are central to healthy bone resorption and remodeling, and suppresses PTH, a hormone that draws calcium and phosphorous from bones to maintain adequate serum concentrations.(31) Vitamin D deficiency can elevate PTH concentration, also known as secondary hyperparathyroidism, which has negative effects on bone mineralization (Figure 2).

Figure 2. The Role of Vitamin D in Calcium and Phosphate Homeostasis.
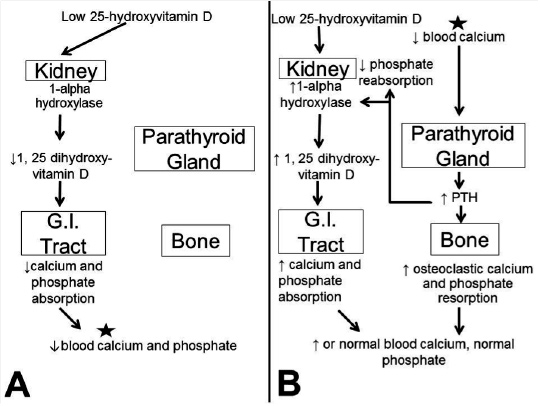
In low vitamin D states (A), decreased levels of 1,25-dihydroxyvitamin D lead to decreased calcium and phosphate absorption in the small intestine. Low blood calcium concentration (continued in B) stimulates increased PTH release from the parathyroid gland leading to: increased calcium and phosphate resorption from bone, increased phosphate secretion in the kidney and up-regulated 1-alpha hydroxylase in the kidneys. Increasing 1-alpha hydroxylase levels increases the amount of the active form of vitamin D and causes increased calcium and phosphate absorption in the GI tract. Ultimately, calcium and phosphate concentrations are maintained at adequate levels, though potentially at the expense of bone mass.

Vitamin D deficiency is associated with rickets and osteomalacia, diseases of poor bone formation, and can contribute to osteoporosis, a disease of increased bone resorption. Rickets, which involves the failure of bone tissue mineralization prior to growth plate fusion,(74) occurs most commonly in children with a vitamin D nutritional deficiency. Infants who are exclusively breastfed, live in northern climates or who are born to dark-skinned mothers are at particularly high risk.(75)(76)(77) In the presentation of rickets, vague symptoms, such as growth failure, lethargy, irritability, increased respiratory infections and hypocalcemic seizures, can be present in the months before the characteristic bowing of the legs and bone pain with ambulation.(78)(79)(80)
Osteomalacia, which typically occurs following mineralization of the growth plates in adults with 25(OH)D concentrations <10 ng/mL,(4) may often present subtly as chronic muscle aches and a waddling gait.(81) In osteoporosis, low vitamin D and calcium concentrations result in elevated PTH, which increases bone resorption and cortical bone loss,(82) and thus increases bone fragility and fracture risk.(83)
High doses of vitamin D may increase fracture risk in elderly populations.
Despite the apparent benefit of daily supplementation, some studies have demonstrated that high doses of vitamin D (300,000-600,000 IU of vitamin D2 and D3) may be detrimental to bone health and increase fracture risk in elderly populations.(88)(89) Thus, while vitamin D appears to prevent fractures, high doses of vitamin D must be used with caution to prevent adverse outcomes. Studies evaluating physical function and fall risk were more variable, with some(90)(91)(92) but not all(88)(93)(94) reporting positive results.
Vitamin D Supplementation and Lowered Mortality
Vitamin D supplementation also appears to play a role in reducing overall mortality. A meta-analysis evaluating vitamin D supplementation in 18 randomized, controlled trials found a 7% relative risk reduction for death.(95) However, results varied in other analyses: a Cochrane Review found a mortality benefit only with vitamin D3 supplementation, as opposed to vitamin D2 or other analogues.(96). Still other analyses found no association between vitamin D and mortality(97) aside from a mild decrease in mortality in the elderly.(99)A reason for this variability may be demonstrated by a population-based study involving data from more than 1,200,000 members of a health maintenance organization (HMO).(99) While this study found serum 25(OH)D concentrations between 20-36 ng/mL to be associated with the lowest risk of mortality and morbidity, the hazard ratio increased at both low levels of 25(OH)D (1.88 and 1.25 in subjects with 25(OH)D concentrations <10 ng/mL and 10-20 ng/mL, respectively) and high 25(OH)D concentrations (1.13 in subjects with 25(OH)D levels >36 ng/mL).
The apparent effect of vitamin D on mortality, coupled with the presence of the vitamin D receptor (VDR) in tissues throughout the body, suggests that vitamin D may play a role in other extra-skeletal diseases. For example, epidemiologic data suggest that vitamin D deficiency increases the risk of cancer in adults.(100)(101)(102)(103)(104) Vitamin D has several functions that support this connection (e.g., blocking the cell cycle, slowing cellular growth, promoting apoptosis, modulating angiogenesis)(105) and in vitro models have found vitamin D to reduce cancer growth(106) and act synergistically with some chemotherapeutic agents.(107)
Though more conclusive clinical trials are underway to establish the role of vitamin D in treating and preventing cancer, the results to date are mixed. While some researchers found that vitamin D had no impact on cancer risk(30)(108) and mortality,(109) or even worse outcomes in pancreatic cancers,(110)(111)(112) higher serum 25(OH)D levels at the time of diagnosis are generally associated with improved outcomes.(113) The evidence is strongest for the role of vitamin D in colorectal cancer, where mortality is inversely related to serum 25(OH)D concentration and males >50 years-old with the highest vitamin D intake (>645 IU/day) have a reduced risk.(114) Though another large study showed no change in colorectal or breast cancer incidence in postmenopausal women receiving 400 IU/day with calcium for seven years,(108), this study was limited by calcium consumption in both groups, non-adherence to daily vitamin D and a relatively low dose of vitamin D.(115)(116)
A role for vitamin D in infectious disease, in particular respiratory illness, is suggested by the presence of the VDR and enzyme CYP27B1 on immune cells,(117)(118) and on bronchial and pulmonary epithelial cells.(119)(120) The VDR and CYP27B1 are up-regulated following the ligation of toll-like receptors by extracellular pathogens,(121) thus implicating vitamin D in innate immunity.(122) By binding the VDR, calcitriol induces endogenous antimicrobial peptides(123)(124)(125) and up-regulates nitric oxide synthase,(126) which increases bacterial killing by macrophages.(127) Calcitriol also reduces inflammation by suppressing pro-inflammatory cytokines(128)(129) and up-regulating anti-inflammatory cytokines such as IL-10.(130) Vitamin D could, thus, potentially reduce the severity and morbidity associated with the inflammatory responses following infections such as influenza, pneumonia and sepsis.(131)(132)(133)(134)
While observational studies have consistently linked vitamin D deficiency to respiratory tract infections (RTI) and influenza,(135)(136)(137)(138)(139)(140) tuberculosis (TB),(141)(142) chronic obstructive pulmonary disease exacerbations,(143)(144) CF(145)(146) and sepsis,(147) clinical trial results have been inconsistent. A systematic review of 38 randomized, controlled studies that measured infectious endpoints following vitamin D administration (e.g., disease incidence and severity, mortality, time to disease resolution or recurrence) found at least one positive endpoint in 10 of 16 trials evaluating RTI and RTI-associated clinical conditions, 8 of 12 trials measuring TB treatment and prevention, 0 of 3 trials evaluating pneumonia, and 1 of 3 trials studying infections and antibiotic use.(148) Thus, though the role of vitamin D in respiratory infections appears promising, the variability in results, likely due to both variables in study design and response to vitamin D supplementation, makes it difficult to form conclusions about vitamin D in infectious disease.
Vitamin D Supplementation and Cardiovascular Disease
Some researchers have reported that vitamin D supplementation appears to improve cardiovascular disease (CVD) markers, including glucose intolerance,(149)(150) insulin sensitivity,(151) hypertension,(152)(153)(154) endothelial function and B-type natriuretic peptide (BNP).(155) However, other studies have shown these and additional markers of CVD to be unaffected by vitamin D.(156)(157) While the data are not convincing for a role for vitamin D in CVD, serum 25(OH)D concentration <10 ng/mL is associated with increased in-hospital mortality in patients with acute coronary syndrome(158) and a recent meta-analysis found an inverse relationship between circulating 25(OH)D concentration and CVD risk up to 20 ng/mL.(159)It is possible that the variability in research results may be due to the differences in the vitamin D status and response to vitamin D in each of the studies. For example, Sugden et al.(153) found insulin sensitivity to be improved only in patients who achieved an increase in 25(OH)D concentration >4.4 ng/mL. similarly, studies using high doses of vitamin D, a 300,000 IU dose of vitamin D3 demonstrated improvements in insulin sensitivity in women with polycystic ovarian syndrome (PCOS),(160) while doses between 100,000 IU-200,000 IU of vitamin D showed no change in patients with type 2 DM.(154)(157)
Findings suggest that vitamin D is highly involved in fetal development. Vitamin D deficiency at 18 weeks of pregnancy was associated with various impairments (e.g., impaired lung development, neurocognitive difficulties, increased risk of eating disorders, lower peak bone mass) in the offspring of 901 mothers.(161) Vitamin D status is also important to mothers during pregnancy, as low levels of vitamin D are associated with higher rates of gestational diabetes and pre-eclampsia.(162)
After evaluating the evidence supporting the numerous potential roles of vitamin D in the body, the Food and Nutrition Board (FNB) found insufficient evidence to link vitamin D with any measures beyond bone health.(40) This conclusion was supported by the Agency for Healthcare Research and Quality following the review of nearly 250 studies.(163)
How Much Vitamin D Does The Body Need?
Though vitamin D can be naturally acquired from sun exposure (vitamin D3) and through diet (vitamin D3 or D2), vitamin D supplementation is often necessary to prevent vitamin D deficiency in adults. The FNB has created the Dietary Reference Intakes (DRIs) for vitamin D and other nutrients in order assess the adequate intake of each nutrient in healthy individuals. The DRI varies by age and gender and can include: (1) the Recommended Dietary Allowance (RDA), or average daily intake needed to meet the requirements of 97-98% of healthy people; (2) Adequate Intake (AI), or the amount of a nutrient that ensures nutritional adequacy when an RDA cannot be developed; and (3) Tolerable Upper Intake Level (UL), or the maximum daily amount of a nutrient unlikely to cause adverse events.(40)
The RDA for vitamin D represents the daily intake of vitamin D sufficient to maintain serum 25(OH)D concentrations >20 ng/ml and increases with age, from 600 IU/day for ages 1-70 and 800 IU/day for 70 and older.(32) The Endocrine Society guidelines on vitamin D suggest higher intakes of vitamin D (1500-2000 IU) for most adults with a goal serum 25(OH)D >30 ng/mL. However, higher doses of vitamin D are frequently required for vitamin D-deficient children or adults. The UL increases by age as well, from 1000 IU/day for ages 0-1 year to 2500 IU/day for 1-3 years to 3000 IU/day for 3-8 years to 4000 IU/day for >9 years (Table 2).

Table 2. Prevention and Treatment of Vitamin D Deficiency.*
| Age Range | RDA | Treatment | UL |
|---|---|---|---|
| 0-1 yr | Loading: 6 wk of 2000 IU/d or 50,000 IU once a wk Maintenance: 400-1000 IU/d |
1000 IU | |
| 1-3 yr 3-8 yr 9-18 yr |
600 IU | Loading: 6 wk of 2000 IU/d or 50,000 IU once a wk Maintenance: 600-1000 IU/d |
2500 IU 3000 IU 4000 IU |
| >18 yra | 600-800 IU | Loading: 8-12 wk of 6000 IU/d or 50,000 IU once a wk Maintenance: 1000-2000 IU/d |
4000 IU |
aIncludes pregnant and lactating individuals
*Adapted from the Endocrine Society's Guideline on Evaluation, Treatment and Prevention of Vitamin D Deficiency(28) and the Institute of Medicine DRI for Calcium and Vitamin D.(32)
Abbreviations: d, days; RDA, Recommended Daily Amount; UL, Tolerable Upper Intake Level;
wk, weeks; yr, years.
The Food and Nutrition Board (FNB) develops the Dietary Reference Intakes (DRIs) to identify how much of a nutrient is necessary for sufficiency in healthy individuals. A DRI may contain: the Recommended Dietary Allowance (RDA), or the daily intake of a nutrient needed to meet the requirements in 97-98% of healthy people, and the Tolerable Upper Intake Level (UL), or the maximum daily amount of a nutrient unlikely to cause adverse events.(40) Regimens to treat vitamin D deficiency vary by age, and typically involve daily or weekly high-dose vitamin D followed by a maintenance dose once serum 25(OH)D concentrations are >30 ng/mL. Certain populations, such as those who are obese, have malabsorption syndromes or take medications affecting vitamin D metabolism, may require doses of vitamin D higher than the UL in order to achieve and maintain sufficiency.

Treatment And Prevention Of Vitamin D Deficiency
Adequate sun exposure remains the primary means of preventing vitamin D deficiency.(28) Sun exposure can be quantified as a minimal erythemal dose (MED), or the amount of time in the sun for the skin to turn pink. One MED is equivalent to ingesting 10,000-25,000 IU of vitamin D3 and can last twice as long in the blood as ingested vitamin D.(164)
Various factors affect vitamin D production by UV radiation exposure, making it difficult create guidelines for adequate sun exposure. For example, cloud cover, pollution and shade can block up to 60% of UV radiation(74) and sunscreen blocks a majority of vitamin D-producing radiation.(165) It has been suggested that 15-30 minutes of sun exposure between 10 AM and 3 PM at least twice a week without sunscreen may lead to sufficient vitamin D synthesis.(166) Similar use of commercial tanning beds can also be effective. However, it is important to recognize the potential carcinogenic effects of UV radiation(165) and it is not known whether adequate sun exposure for vitamin D sufficiency can be obtained without increasing cancer risk.
Individuals with inadequate sun exposure should supplement their diet with vitamin D-containing foods. Vitamin D2 and D3 are from different sources: vitamin D2 is formed from UV irradiation of ergosterol in yeast or fungi, while vitamin D3 is manufactured by irradiating 7-dehydrocholesterol (the same precursor found in the skin) in lanolin or converted from cholesterol.(3) The best natural food sources of vitamin D3 are from fatty fish (e.g., salmon, tuna, mackerel) and fish liver oils.(40) Certain mushrooms can also provide vitamin D2.(167)(168)
In the U.S., fortified foods are the best source of dietary vitamin D.(40)(168) Nearly all infant formulas and milk are fortified, which has nearly eliminated rickets since its introduction in 1930.(40) A list of vitamin D-containing foods as published by the U.S. Department of Agriculture's (USDA) Nutrient Data Laboratory(169) is provided in Table 3, though it is important to recognize that the amounts of vitamin D in these products can vary widely from what is advertised.(170)(171) The best natural food sources of vitamin D are from fatty fish and fish liver oils for vitamin D3(40) and certain mushrooms for vitamin D2.(167)(168)

Table 3. Natural and Fortified Sources of Vitamin D.*
| Source | Amount of Vitamin D |
|---|---|
| Sun exposure | 10,000 IU-25,000 IU of vitamin D3 per MED |
| Vitamin D-containing foods (IU/100 g) Fish Atlantic mackerel, raw Atlantic cod, raw Bluefin tuna, raw Canned salmon Canned sardines Cod liver oil Pickled herring Meat Beef liver, raw Dairy/eggs Butter, salted Cheddar cheese Egg, scrambled swiss cheese Fungi Portabella mushrooms, raw shiitake mushrooms, raw |
643 IU 36 IU 227 IU 547 IU 193 IU 10,000 IU 130 IU 49 IU 60 IU 24 IU 72 IU 20 IU 10 IU 18 IU |
| Vitamin D-fortified foods (100 g) Fortified 2% milk Fortified American cheese Fortified yogurt |
40 IU/ 98 IU per cup 274 IU/ 52 IU per slice 52 IU/ 127 IU per cup |
*Adapted from the USDA website(169)
Abbreviations: MED, minimal erythemal dose.

Treatment of vitamin D deficiency varies by age and typically calls for daily or weekly high-dose vitamin D followed by a maintenance dose once serum 25(OH)D concentrations are >30 ng/mL (Table 2.).(28) For patients <1 year-old, treatment involves six weeks of vitamin D at either 2000 IU/day or 50,000 IU once a week followed by a 400-1000 IU/day maintenance dose. Patients ages 1-18 years should receive the same six weeks of high-dose treatment followed by a maintenance dose of 600-1000 IU/day (28). Vitamin D deficient adults should receive 8-12 weeks of treatment with vitamin D at either 50,000 IU/week or 6000 IU/day followed by a maintenance dose of 1500-2000 IU/day. Certain populations, such as those who are obese, have malabsorption syndromes, or take medications that affect vitamin D metabolism, should receive 6000-10,000 IU of vitamin D daily followed by a maintenance dose of 3000-6000 IU/day.
While these recommendations do not specify whether vitamin D2 or D3 should be used, there is some evidence to suggest that vitamin D2 is less potent than vitamin D3 at high doses (see below: What factors influence the response to supplementation?). It is also possible that single, large doses of vitamin D may be more efficacious than daily doses. Large, bolus doses have demonstrated better adherence compared to daily and monthly dosing regimens(172) and it has been shown that doses of vitamin D distributed into daily, weekly and monthly doses can sustain the same circulating concentrations of 25(OH)D over an equivalent period of time.(173) Large, oral, bolus doses appear to be most effective when >200,000 IU and when using cholecalciferol rather than ergocalciferol.(174)
What Factors Influence The Response To Supplementation?
Making generalizations about how vitamin D intake in food and supplements may impact serum 25(OH)D concentration can be problematic due to the wide variation in response to vitamin D supplementation.(175)(176) For example, body composition can affect vitamin D supplementation since fat can sequester vitamin D from circulation.(51) Baseline vitamin D status also impacts the response to supplementation — vitamin D sufficient individuals achieve a smaller increase in 25(OH)D concentration compared to deficient individuals receiving supplementation.(177) Genetic variations in the vitamin D binding protein (DBP)(178) and vitamin D receptor (VDR)(179) also impact individual responses to vitamin D supplementation. Vitamin D can interact with certain medications that can increase its catabolism (such as corticosteroids and several anti-convulsants)(180)(181)(182)(183) or reduce its absorption (such as the cholesterol-lowering medication cholestyramine and the weight-loss drug Orlistat.(48)(49)
The formulation of vitamin D (vitamin D2 vs. D3) may also impact the response to vitamin D supplementation. Though both vitamin D2 and D3 have been used for treating vitamin D deficiency in the past and Holick et al.(184) found 1000 IU doses of vitamin D2 and D3 to be equivalent for supplementation, some studies suggest that vitamin D3 may be more effective than vitamin D2 for normalizing vitamin D status.(185)(186)(187) These studies tended to evaluate higher doses than those that found vitamin D2 and D3 to be equivalent. One study analyzing daily doses at the UL for adults (4000 IU) found two weeks of vitamin D3 to be 1.7 times more effective than the same regimen of vitamin D2 at raising 25(OH)D levels.(186) A systematic review of large, bolus doses >100,000 IU concluded that vitamin D3 provided a higher and more sustained increase in 25(OH)D concentration relative to equivalent doses of vitamin D2 and more consistently suppressed PTH concentration.(185). Houghton and Vieth(188) suggest that vitamin D3 may bind with higher affinity to DBP in plasma than vitamin D2, thus enhancing its half-life.
Vitamin D Toxicity
Vitamin D toxicity is thought to be dictated by 25(OH)D concentration rather than 1,25(OH)2D concentration.(27) While 1,25(OH)2D has a short half-life and is tightly-regulated by feedback mechanisms, there are no known regulatory mechanisms for the conversion of vitamin D3 to 25(OH)D3(189)(190) and, in fact, 25(OH)D can bind to the vitamin D receptor, albeit at lower affinity than 1,25(OH)2D. In two case studies of vitamin D intoxication, patients had 25(OH)D concentrations far above the normal range yet only slightly elevated 1,25(OH)2D concentrations.(7) This observation is likely because low PTH concentration suppressed the conversion of 25(OH)D to 1,25(OH)2D.
Increased risk for vitamin D toxicity is thought to begin at 25(OH)D concentrations >150 ng/mL (191) by some, and potentially even >200 ng/mL by others.(192)(193) Hypercalcemia is responsible for producing most of the symptoms of vitamin D toxicity, which can be non-specific (e.g., nausea, vomiting, headache, anorexia, metallic taste, constipation, frequent urination).(194)(195) Over a longer period of time, elevated calcium leads to vascular and tissue calcification(40) such as nephrocalcinosis and pancreatitis.(195) Hyperphosphatemia is also associated with vitamin D toxicity and was found to be a major contributor to vascular calcifications in mouse models.(196)
Toxicity typically occurs following supplementation with high doses of vitamin D for prolonged periods of time.(40) There is no consensus as to what oral dose leads to intoxication, though Vieth reported that chronic consumption of approximately 40,000 IU/day is necessary to cause toxicity.(197) Lethal doses of vitamin D have been reported as high as 840,000 IU/kg.(198) In a systematic review of large, oral doses of vitamin D, no adverse events were associated with doses <200,000 IU and doses as high as 600,000 IU of vitamin D3 were generally very well-tolerated.(185) Of note, excessive sun exposure will not cause vitamin D intoxication since heat from the sun breaks down previtamin D and vitamin 3 as it is formed in the skin.(199)
Hypercalcemia is responsible for producing most of the symptoms of vitamin D toxicity.
Future Directions
Moving forward, vitamin D research must focus on reducing the high level of variability between study results. One means of achieving this is by improving the accuracy with which vitamin D status is measured. As described above, a majority of 25(OH)D in circulation is tightly bound by vitamin D binding protein (DBP), while only approximately 15% of the total 25(OH)D (that which is free in plasma and loosely bound to albumin) is bioavailable and freely accessible to cells.(13) Though free vitamin D and albumin concentrations are largely fixed across populations, DBP concentration and binding affinity vary by race, genetics(200)(201) and certain medical conditions. For example, diseases such as CF(202) and CKD are known to have low DBP concentrations.
These variations in DBP may contribute to the well-documented differences in vitamin D status (as determined by measuring total serum 25(OH)D concentration) between different races and disease processes, since free levels of bio-available vitamin D tend to be relatively constant across populations.(203) Recognizing the influence of variations in the DBP, in addition to the ability to measure free vitamin D, may allow for more accurate measurement of baseline and post-treatment vitamin D status, as well as better stratification of cohorts within studies.
While recognizing and accounting for variations in DBP may be essential to the more accurate assessment of vitamin D status, stratifying groups by the genotype of the vitamin D receptor (VDR) may also reduce the variability of study results in the future. Martineau et al.(204) found, for example, that the efficacy of vitamin D on treatment of TB was dependent on the genotype of the Taq1 VDR. Though performing genotyping in all studies may be unrealistic, stratifying populations by known or common genotypic variants of the VDR may be a reasonable approach to obtaining more homogenous populations that demonstrate less variability in their response to vitamin D supplementation.
In addition to advancements in how we measure vitamin D, there are several robust studies underway that hope to add to the body of evidence surrounding vitamin D in health and disease. One prominent example is the Vitamin D and Omega-3 Trial (VITAL, http://www.vitalstudy.org/), which is evaluating 25,875 individuals in the U.S. as to whether daily doses of 2,000 IU of vitamin D3 or omega-3 fatty acids reduce the risk of diseases such as cancer, heart disease and stroke in individuals with no prior history of disease. Large, prospective trials such as VITAL will be essential to the effort to more accurately and conclusively define the roles of vitamin D.
Conclusions
With vitamin D deficiency prevalent in both healthy and diseased populations, it is important for clinicians to suggest measures for patients to prevent vitamin D sufficiency (such as adequate time outdoors, vitamin D fortified foods and daily supplements) and to identify populations at risk for vitamin D deficiency. At-risk individuals should be screened for vitamin D deficiency by having their serum 25(OH)D concentrations measured. Though controversy exists as to what concentration of serum 25(OH)D is sufficient, evidence suggests that serum 25(OH)D concentrations >30 ng/mL optimize the physiologic processes regulated by vitamin D concentration. While the role of vitamin D sufficiency appears to reduce mortality and is well-established in bone health, further randomized controlled trials are necessary to validate other physiological functions of vitamin D in the body.
Recommended Articles
Measuring Vitamin D Status
- Holick MF. Vitamin D status: measurement, interpretation, and clinical application. Annals of epidemiology. 2009;19(2):73-8.
- Powe CE, Ricciardi C, Berg AH, et al. Vitamin D-binding protein modifies the vitamin D-bone mineral density relationship. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26(7):1609-16.
- Schwartz JB, Lai J, Lizaola B, et al. A comparison of measured and calculated free 25(OH) vitamin D levels in clinical populations. The Journal of clinical endocrinology and metabolism. 2014;99(5):1631-7.
- Weintraub sJ. Vitamin D-binding protein and vitamin D in blacks and whites. The New England journal of medicine. 2014;370(9):878.
Vitamin D In Disease
- Ashraf AP, Alvarez JA, Dudenbostel T, et al. Associations between Vascular Health Indices and serum Total, Free and Bioavailable 25-Hydroxyvitamin D in Adolescents. Plos one. 2014;9(12):e114689.
- Kearns MD, Alvarez JA, Seidel N, Tangpricha V. Impact of Vitamin D on Infectious Disease: A systematic Review of Controlled Trials. The American journal of the medical sciences. 2014.
- Hoffmann MR, Senior PA, Mager DR. Vitamin D Supplementation and Health-Related Quality of Life: A systematic Review of the Literature. Journal of the Academy of Nutrition and Dietetics. 2015.
- Ying HQ, Sun HL, He BS, et al. Circulating vitamin D binding protein, total, free and bioavailable 25-hydroxyvitamin D and risk of colorectal cancer. scientific reports. 2015;5:7956.
Vitamin D supplementation and Vitamin D-containing Foods
- Kearns MD, Alvarez JA, Tangpricha V. Large, Single-Dose, Oral Vitamin D supplementation in Adult Populations: A Systematic Review. Endocrine practice: official journal of the American College of Endocrinology and the American Association of Clinical Endocrinologists. 2013:1-36.
- U.S. Department of Agriculture ARS. USDA National Nutrient Database for Standard Reference Release 27. Nutrient Data Laboratory Home Page, http://wwwarsusdagov/ba/bhnrc/ndl. 2014.