Management of Non-Valvular Atrial Fibrillation – The New Era of Oral Anticoagulants
Course Authors
Muhib Khan, M.D., and Karen Furie, M.D., M.P.H.
Dr. Khan is Assistant Professor and Dr. Furie is Professor and Chair, Department of Neurology, The Warren Alpert Medical School of Brown University, and Neurologist-in-Chief, Rhode Island Hospital, The Miriam Hospital and Bradley Hospital, Providence, RI.
This Cyberounds® program was edited by Mark Crowther, M.D., M.Sc., Professor of Medicine, Pathology & Molecular Medicine and Clinical Epidemiology & Biostatistics, Associate Chair, Department of Medicine, Leo Pharma Chair in Thromboembolism, McMaster University School of Medicine, and Vice President, Research, St Joseph`s Healthcare System, Chief of Laboratory Medicine, Hamilton Health Sciences and St Joseph`s Healthcare, Toronto, Canada.
Within the past 12 months, Drs. Furie and Khan report no commercial conflicts of interest; Dr. Crowther has been on advisory boards for Leo Pharma, Pfizer, Bayer, Boehringer Ingelheim, Alexion, CSL Behring, Portola, Viropharm and AKP Americas, prepared educational materials/presentations for CSL Behring, Celgene, Pfizer and Octapharm, and his institution has received research grant support from Boehringer Ingelheim, Leo Pharma, Octapharm and Pfizer.
Albert Einstein College of Medicine, CCME staff, and interMDnet staff have nothing to disclose.
This activity is certified for cardiovascular medicine, hematology, neurology, primary care (internal medicine, family practice and women’s health), physician assistants, nurse practitioners, nurses, pharmacists, and for all other health professionals interested in the management of atrial fibrillation stroke prevention.
This CME activity has been peer-reviewed by Ileana L. PiA?a, M.D., M.P.H., Professor of Medicine & Epidemiology and Population Health, Albert Einstein College of Medicine, and Associate Chief for Academic Affairs, Division of Cardiology, Montefiore Medical Center, Bronx, New York.
This activity is made possible by an unrestricted educational grant from Boehringer Ingelheim
Estimated course time: 2 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 2.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Assess the thromboembolic risks associated with atrial fibrillation and apply this knowledge to patient stroke prevention
Discuss the data emerging from recent clinical trials of prospective pharmacotherapies and strategies for atrial fibrillation stroke prevention including safety, efficacy and side effects
Apply risk stratification in the selection of appropriate therapy (devices, surgeries and pharmacotherapies) for patients with atrial fibrillation.
This presentation may include discussion of commercial products and services.
Atrial fibrillation (AF) is defined as a tachyarrhythmia of supraventricular origin leading to deterioration in mechanical function of the atrium. An electrocardiogram (ECG) shows fibrillatory waves varying in amplitude, shape and timing instead of P waves. These fibrillatory waves lead to an irregular rapid ventricular response.(1) AF can occur with other atrial arrhythmias of which atrial flutter is the most common entity. The most important feature which distinguishes AF from other atrial arrhythmias is the absence of P waves.(2)(3)
Classification
AF is a dynamic process leading to changes in its duration over time. Therefore, a better understanding of the classification of AF is imperative for the treating physician. The first-detected episode can proceed along different pathways. It can be self-limited without any recurrence in which case it is termed lone AF. It can adopt a recurrence pattern with intervening sinus rhythm termed as paroxysmal AF. If it persists for seven days, it is termed persistent.(4) It is important to note that the AF duration and its persistence can evolve over time either due to ongoing pathophysiological process or treatment. The exact length of a qualifying event for classification as atrial fibrillation is >30 seconds, although this has become controversial recently.(5)
Atrial fibrillation can be further divided into valvular vs. non-valvular atrial fibrillation. Valvular atrial fibrillation is defined as atrial fibrillation secondary to structural heart disease involving the valves, commonly the mitral valve. Other structural heart diseases such as dilated cardiomyopathy, congenital heart disease, atrial septal defects, restrictive cardiomyopathies, cardiac tumors, constrictive pericarditis, mitral valve prolapse with or without mitral regurgitation, calcification of the mitral annulus, cor pulmonale and idiopathic dilation of the right atrium are also associated with a high incidence of AF. Non-valvular atrial fibrillation is defined as atrial fibrillation without evidence of structural valvular heart disease preferably screened by an echocardiogram.(2) In this Cyberounds®, we focus on non-valvular atrial fibrillation.
Epidemiology and Risk Factors
Atrial fibrillation prevalence is associated with age. Over-all population prevalence is 0.4% to 1% but increased to 10% by 80 years of age.(6)(7)(8) Prevalence is higher among men even when adjusted for age.(8) Approximately 60% of AF patients over 75 years are, however, female. Caucasians are at higher risk of developing AF as compared to African-Americans even when adjusted for presence of heart failure.(9)
Hypertension is the most important risk factor responsible for AF.(11) Heart failure, valvular heart disease, myocardial infarction and diabetes significantly increase the likelihood of AF.(10)(11) Left atrial enlargement, left ventricular fractional shortening and left ventricular wall thickness detected on echocardiography are predictors of AF.(12)
Hypertension is the most important risk factor responsible for AF.
Hyperthyroidism has been implicated as a predisposing condition and 2-30% of patients with overt hyperthyroidism have AF.(13) Individuals with subclinical hyperthyroidism have been found to have a greater incidence of AF compared with those who have normal thyroid function.(14)
Obesity is a risk factor even when adjusted for cardiovascular risk factors. With adjustment for echocardiographic left atrial diameter, BMI is no longer associated with AF risk. Therefore, obesity is thought to be a modifiable risk factor for AF, mediating its effect mainly by left atrial dilatation.(15) Cigarette smoking is a significant risk factor in women.(11) Alcohol consumption at sufficiently high amounts is related to occurrence of AF.(16)(17)
Multiple genetic polymorphisms have been implicated in the pathogenesis of atrial fibrillation. These genes mostly encode for proteins important for the integrity of cardiacmyocyte structure and normal physiology.(18)
Pathophysiology
AF is the result of complex interaction between a trigger of rapidly firing ectopic foci and abnormal atrial tissue maintaining the arrhythmia. Rapid ectopic foci, usually located in the left atrium and proximal parts of pulmonary veins, play an important role in the initiation of AF.(19) The exact mechanism involved in the production of ectopic activity in these areas is unclear. Atrial tissue capable of maintaining the arrhythmia is the key for the perpetuation of AF.(20) Conduction slowing and shortening in the atrial tissue makes it susceptible to maintaining AF.(21) AF can cause progressive changes in atrial electrophysiology, facilitating the perpetuation of the arrhythmia. This concept of electrical remodeling has been demonstrated in humans, especially in cardioversion studies.(22)(23)(24)
Stroke associated with AF is attributed to embolism of the thrombus in the left atrium (LA), the pathogenesis of which is complex.(25) Thrombus most frequently forms in the left atrial appendage (LAA).(26) This thrombus is a result of stasis, endothelial dysfunction and a hypercoagulable state. Stasis results from the decreased emptying of the LAA due to loss of organized mechanical contraction during the cardiac cycle, as evidenced by the reduced LAA flow velocities.(27) Moreover, AF seems to promote a hypercoaguable state and has been associated with biochemical markers of coagulation and platelet activation.(28) In summary, complex electrophysiological and thromboembolic processes lead to embolic events in AF.
Stroke associated with AF is attributed to embolism of the thrombus in the left atrium (LA)
Thrombin Biology and Pathophysiology
The coagulation process (Figure 1) includes three overlapping phases. In the initiation phase, tissue factor (TF)-expressing cells are exposed to the coagulation factors initiating thrombosis. Platelets are recruited and adhere to the site of injury. The TF/FVIIa complex activates coagulation factors IX to IXa and X to Xa, and trace amounts of thrombin (Factor IIa) are generated. In the amplification phase, this small amount of FIIa is a signal for further platelet activation and aggregation. FIIa activates FV, FVIII and FXI. In the propagation phase, FVIIIa forms a complex with FIXa (Xase), and FVa forms a complex with FXa (prothrombinase) on the platelet surface, which accelerate the generation of FXa and thrombin, respectively. When FXa associates with FVa, it is protected from tissue factor pathway inhibitor (TFPI) and antithrombin (AT). In the propagation phase, a burst of FIIa is generated, which is sufficient for the clotting of soluble fibrinogen into a fibrin meshwork. A thrombus is eventually formed.(29)

Figure 1. Physiology of Coagulation Cascade.
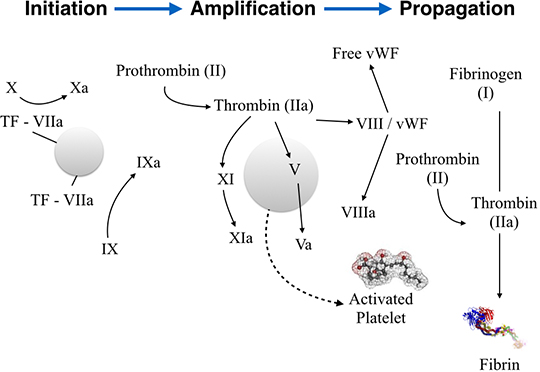

Vitamin K antagonists (e.g., warfarin) exert their anticoagulant effects by reducing the functional levels of factors II, VII, IX and X. New oral anticoagulants, however, are highly specific for a single clotting enzyme. Most of the agents target thrombin or factor (F) Xa. Targeting thrombin is important since it not only converts fibrinogen to fibrin, but also, by activating FXIII, renders fibrin resistant to fibrinolysis. Another target in the coagulation cascade is FXa. FXa inhibitors are small molecules that bind to the active site of factor Xa in a reversible fashion.(30)
Evaluation of patients suspected of AF consists of history, clinical examination and ECG recording. ECG recording can be a routine 12-lead ECG, bedside telemetry, ambulatory Holter monitoring and, recently, cardiac event monitors. AF should be characterized as lone, paroxysmal or persistent, as explained earlier.
History should be focused on identifying symptoms of AF, as well as the underlying etiology and the risk factors associated with AF. Patients should be asked thoroughly about symptoms suggestive of transient ischemic attack (TIA), stroke and chest pain. Since genetic associations have been identified, family history is also important.
The physical examination findings of irregular pulse, irregular jugular venous pulsations and variation in the intensity of the first heart sound suggest AF. Examination should also focus on identifying valvular heart disease and heart failure (HF).
ECG documentation by at least a single-lead recording during the arrhythmia is needed for AF diagnosis. A portable ECG recording tool such as Holter monitor, event recorder or loop recorder may help establish the diagnosis in cases of paroxysmal AF. Recently, various studies have highlighted the higher yield of atrial fibrillation detection with longer monitoring and newer devices. The yield varies (5%–28%) depending on the choice of monitoring devices, study population, stroke characteristics, interval of monitoring from stroke onset, duration of cardiac monitoring and most importantly the definition of paroxysmal atrial fibrillation.(31)
A chest radiograph helps detect pulmonary pathology and evaluates the pulmonary vasculature. Transthoracic echocardiography (TTE) is needed to assess left atrium (LA) and left ventricle (LV) dimensions, LV wall thickness and function, occult valvular or pericardial disease. Left atrial appendage (LAA) imaging should be sought and usually needs transesopahgeal echocardiogram (TEE).(32)(33) However, TEE is invasive with associated risk of complications. Therefore, magnetic resonance imaging (MRI) has emerged as a powerful tool to detect thrombus and is significantly less invasive. Visualization of appendage thrombus has been demonstrated on MRI using various techniques. For enhanced detection, a combination of these imaging sequences is used.(34)(35)
Treatment
Treatment for AF is multimodality and focuses on three important aspects: rate control, rhythm control and prevention of thromboembolism. Societies all over the world have published guidelines for the optimal treatment of AF patients. Instead of discussing each society's guideline individually, we provide a comparative analysis of these guidelines with a focus on differences. Before we discuss the guidelines, it is important to understand some key concepts of risk stratification for thromboembolic events and bleeding as related to treatment.
Magnetic resonance imaging (MRI) has emerged as a powerful tool to detect thrombus.
Risk Stratification
The absolute risk of stroke varies significantly among AF patients according to age and associated comorbidities. Different scores have been developed and validated to predict this risk in an individual.
The most widely used score is CHADS2. CHADS2, an acronym for Congestive heart failure, Hypertension, Age ??JPY75 years, Diabetes mellitus, and prior Stroke or TIA. The CHADS2 score was derived from independent predictors of stroke risk in patients with nonvalvular AF. The score assigns 1 point each for congestive heart failure, hypertension, age ??JPY75 years and diabetes mellitus, and 2 points for prior stroke or TIA. (36) In a large validation cohort, CHADS2 score of 0 had a thromboembolic rate of 0.49 versus 1.52 for CHADS2 score=1, 2.50 for CHADS2 score=2, 5.27 for CHADS2 score=3, 6.02 for CHADS2 score=4, and 6.88 for CHADS2 score=5 or 6. A limitation of the CHADS2 score applies to secondary prevention in patients with prior stroke or TIA and no other risk factors.(36)
The CHA2DS2-VASc index further refines the risk calculation of CHADS2 by including additional variables: Congestive heart failure/left ventricular dysfunction, Hypertension, Age ??JPY75 [doubled], Diabetes, Stroke [doubled] – Vascular disease, Age 65–74 and Sex category [female]).(37) Among patients with CHADS2 score 0, the 1-year event rates can range between 0.84% (CHA2DS2-VASc score 0), 1.75% (CHA2DS2-VASc score 1), 2.69% (CHA2DS2-VASc score 2) and 3.2% (CHA2DS2-VASc score 3).(37)(38)
Hemorrhage risk can also vary among individuals. Bleeding risk tools such as the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile INR, Elderly, Drugs/Alcohol concomitantly), ATRIA (Anticoagulation and Risk Factors in Atrial Fibrillation) and HEMORR2HAGES [Hepatic or renal disease, Ethanol abuse, Malignancy, Older (age ??JPY75 years), Reduced platelet count or function, Rebleeding risk, Hypertension (uncontrolled), Anemia, Genetic factors, Excessive fall risk, and Stroke] have been developed to estimate the likelihood of hemorrhage, but they have low predictive accuracy.(39)(40)(41)
Knowledge of various risk stratification tools helps the clinician understand the treatment guidelines. The American College of Cardiology Foundation/American Heart Association/Heart Rhythm Society (ACCF/AHA/HRS), Canadian Cardiovascular Society (CCS) and American College of Chest Physicians (ACCP) guidelines have recommended the CHADS2 index for primary risk assessment and selection of antithrombotic therapy. The European Society of Cardiology (ESC) recommends the CHA2DS2-VASc for assessment of stroke risk in patients with AF. ESC and CCS guidelines also recommend the use of the HAS-BLED scoring system to evaluate the risk of bleeding.
Comparative Analysis of All Guidelines
Rate Control
Optimal rate control in patients with AF is controversial. The new ACCF/AHA/HRS guidelines state that treatment to achieve strict control of the heart rate is not beneficial compared with lenient control.(42) The European and Canadian recommendations have made similar changes.(43)(44) This change in guidelines is driven by the results of the Rate Control Efficacy in Permanent Atrial Fibrillation (RACE II) study. This study found that strict heart rate control in patients with AF was not beneficial compared with lenient control. A larger proportion of patients treated with the lenient strategy achieved their target heart rate goal, with lower drug doses and fewer drug combinations, resulting in far fewer outpatient visits to achieve the intended target.(45) In general, lenient rate control is more convenient (requires fewer outpatient visits and examinations) and easier to achieve. Thus, lenient rate control might be a reasonable strategy for patients with permanent AF. The ACCF/AHA/HRS, ESC and CCS guidelines have all stated that strict rate control is no longer considered superior to lenient rate control. The updated ACCP guidelines do not comment on a target heart rate and do not make specific recommendations for rate control strategies.(46)
Optimal rate control in patients with AF is controversial.
Rhythm Control Strategy
ACCF/AHA/HRS guidelines find no benefit in a routine rhythm-control strategy for patients with AF and systolic heart failure compared with a rate-control strategy.(42) This recommendation is based on the Atrial Fibrillation and Congestive Heart Failure Trial, which found that a routine rhythm-control strategy did not reduce the death rate from cardiovascular causes compared with a rate-control strategy in patients with AF and congestive heart failure.(47) The CCS recommends a rhythm-control strategy for patients who are symptomatic despite rate-control.(44) (44) Similarly, the ESC recommends rhythm-control therapy for symptomatic patients despite adequate rate control.(43) (43) The ACCP did not provide specific recommendations for rhythm-control therapy in patients with AF.(46) However, they recommend continued anticoagulation in high-risk patients even after sinus rhythm has been restored. This recommendation is based on the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) trial.(48)
|
Clinical Vignette One Mrs. T is a 64-year-old woman referred with new AF. She has no prior medical history, nor history of CHF, no TIA, no DM. She does have drug-controlled hypertension. Should she be placed on a new oral anticoagulant (NOAC) and if so which one? |
|
Clinical Comments Mrs. T is a low-risk patient with a low CHADS 2 score. She can be comfortably managed with aspirin at this point based on the AHA guidelines for low-risk patients. |
Prevention of Thromboembolism
Antiplatelet Agents
ACCF/AHA/HRS guidelines recommend aspirin for low-risk and some moderate-risk patients with AF on the basis of patient preference, estimated high bleeding risk if anticoagulated and limited access to high-quality anticoagulation monitoring. The guidelines also recommend dual-antiplatelet therapy with clopidogrel and aspirin for high-risk patients with AF deemed unsuitable for anticoagulation but added a comment about an increased risk of major bleeding.(42) This recommendation is based on the results from the Atrial Fibrillation Clopidogrel Trial with Irbesartan for Prevention of Vascular Events (ACTIVE W) and Effect of Clopidogrel Added to Aspirin in Patients with Atrial Fibrillation (ACTIVE A) trials.(49)(50)
ECS guidelines, however, recommended that aspirin plus clopidogrel therapy should be limited to patients who refuse oral anticoagulation therapy.(51) The ACCP recommends combination therapy with aspirin plus clopidogrel for patients with AF who are unsuitable for, or who choose not to take, oral anticoagulation therapy.(46) CCS on the other hand does not recommend aspirin plus clopidogrel therapy for patients with AF for whom warfarin is considered unsuitable. In such cases, the CCS recommended the use of dabigatran because it might reduce the risk of stroke at a lower risk of bleeding compared with warfarin.(52) In fact, the CCS states that dabigatran should be preferred in most patients who need antithrombotic therapy for AF. The CCS also noted the similar or increased bleeding risk with aspirin plus clopidogrel compared with anticoagulation with warfarin.
The ACCF/AHA/HRS did not make specific recommendations regarding triple therapy (warfarin + dual-antiplatelet therapy) for patients with AF and stents. They noted, however, that this strategy leads to increased bleeding complications.(53) The ESC recommends that triple therapy might be acceptable in high-risk patients such as those with AF after coronary artery stenting in situations with moderate to high thromboembolic risk. They also suggest duration of treatment for different scenarios. For bare metal stents, one month of triple therapy; drug-eluting stents require 3 months (for the sirolimus, everolimus, tacrolimus group) or 6 months (paclitaxel) of triple therapy.
The ECS also recommends triple therapy in the initial period (3 to 6 months) after an acute coronary syndrome with or without percutaneous coronary intervention.(43) Similary, CCS recommends a period of triple therapy for optimal prophylaxis in patients at a high risk of stroke. The guidelines did not, however, specify the duration of the triple therapy.(52) ACCP also recommends triple therapy during the first month after placement of a bare metal stent or the first 3 to 6 months after placement of a drug-eluting stent.(46)
Anticoagulation
ACCF/AHA/HRS recommends anticoagulation for all patients with nonvalvular AF deemed to be at high risk and many deemed to be at moderate risk for stroke who can receive it safely. They recommend using CHADS2 score for risk stratification.(42) ESC recommends anticoagulation for all patients with nonvalvular AF deemed to be moderate to high risk for stroke. They recommend using CHADS2-VASc score for risk stratification.(51) The CCS recommends anticoagulation for patients with nonvalvular AF at moderate to high risk of stroke. They recommend using CHADS2 for stroke risk assessment and HAS-BLED score for bleeding risk.(52) ACCP recommends anticoagulation for moderate to high-risk nonvalvular AF patients. They recommend using CHADS2 for risk assessment.(46)
|
Clinical Vignette Two Mr. M is a 78-year-old male referred with new AF. He has longstanding, moderately well controlled hypertension, and a TIA six weeks ago that led to a diagnosis of AF. He has had diabetes (DM) for 10 years, with poor control on meds. His calculated creatinine clearance is 65 ml/min. Should he be anticoagulated and, if so, with what? |
|
Clinical Comments Mr. M is a high-risk patient based on the CHADS2 score. The current guidelines recommend anticoagulation for such patients. Because of his good renal function and age less than 80, he is a strong candidate for either warfarin or newer anticoagulants. Since he hasn't had any GI bleeding in the past, he is the kind of patient who would be a prime candidate for dabigatran since it has a superior profile to warfarin in preventing ischemic strokes. |
New Oral Anticoagulants
ACCF/AHA/HRS recommends dabigatran as a useful alternative to warfarin to reduce the risk of stroke and systemic embolism in patients with nonvalvular AF.(42) They also noted that dabigatran should be avoided in patients with severe renal failure (CrCl<15 mL/min), or advanced liver disease (impaired baseline clotting function). Dabigatran 150 mg twice daily is the recommended dose in these guidelines. They also recommend dabigatran 75 mg twice daily for patients with low CrCl (15–30 mL/min) with caution.(53) Focused update to the ESC guidelines published in 2012 recommends dabigatran in preference to warfarin for stroke prevention in AF. The guidelines used the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history or predisposition, Labile international normalized ratio, Elderly, and Drugs/alcohol) bleeding risk scoring system to stratify the recommendations for dose. For a patient with low risk of bleeding (e.g., HAS-BLED score 0 to 2), dabigatran 150 mg twice daily; if a patient has a greater risk of bleeding (e.g., HAS-BLED score JPY3), the ESC guidelines recommend dabigatran 110 mg twice daily.(51)
The CCS recommends that dabigatran should be preferred over warfarin, with the possible exceptions of patients who have a propensity to dyspepsia or gastrointestinal bleeding and those at substantial risk of coronary events. Dose recommended is 150 mg twice daily for most patients and 110 mg twice daily for patient's ??JPY80 years old or patients with a low body weight, decreased renal function or an increased risk of major bleeding.(52) ACCP suggests that dabigatran 150 mg twice daily should be preferred over warfarin with the exception of patients with AF and mitral stenosis, stents or those who experience an acute coronary syndrome. They also noted that clinicians should be aware that no antidote is available for dabigatran.(46)
ACCF/AHA/HRS recommends dabigatran as a useful alternative to warfarin.
Since rivaroxaban and apixaban were approved for use after the publication of these guidelines, societies have published updates to tackle this matter. A scientific advisory from AHA recommends that in patients with nonvalvular AF who are at moderate to high risk of stroke (prior history of TIA, stroke, or systemic embolization or ??JPY2 additional risk factors), rivaroxaban 20 mg/d is reasonable as an alternative to warfarin. They also advise against the use of rivaroxaban in patients with CrCl<15 ml/min.(54) In 2012, the ECS and CCS both published focused updates to their guidelines, recommending rivaroxaban in preference to warfarin for stroke prevention in patients with AF.(51)(55)
The American Heart Association/American Stroke Association (AHA/ASA) recommends apixaban 5 mg twice daily as an alternative to warfarin in patients with nonvalvular AF who have at least one additional risk factor and no more than one of the following characteristics: Age ??JPY80 years, weight ?60 kg or serum creatinine ??JPY1.5 mg/dL. They advise against the use of apixaban in patients with CrCl is <25 mL/min.(54) The ESC and CCS focused updates noted that apixaban is also recommended in preference to warfarin.(51)(55) Later in this Cyberounds® we will discuss the newer anticoagulants in detail and will provide a comparative analysis to guide patient therapy decisions.
Catheter-Based Ablation Therapy
The ACCF/AHA/HRS recommends catheter-based ablation for three types of patients who have: (1) symptomatic, paroxysmal AF in whom >1 antiarrhythmic drug has failed; (2) symptomatic, persistent AF; (3) symptomatic, paroxysmal AF with significant left atrial dilation or significant left ventricular dysfunction. (42) The ESC guidelines recommend catheter ablation as a first-line treatment for some patients.(51) CCS recommends catheter ablation for patients who remain symptomatic during antiarrhythmic drug therapy and for whom rhythm control remains desirable. The CCS also suggests catheter ablation as the first-line therapy for highly selected patients with symptomatic, paroxysmal AF (i.e., patients with a strong intolerance or aversion to antiarrhythmic drugs).(56) The ACCP guidelines do not provide specific recommendations for the use of catheter-ablation therapy in the management of AF.
New Oral Anticoagulants (OAC)
Dabigatran
Dabigatran etexilate is an oral prodrug that is converted to dabigatran, a direct, competitive inhibitor of factor IIa (thrombin). The absolute bioavailability is 6.5%, and the serum half-life is 12 to 17 hours.(57) Eighty percent of dabigatran is excreted renally.
The Randomized Evaluation of Long-Term Anticoagulation Therapy (RE-LY) compared open-label warfarin with two fixed, blinded doses of dabigatran (110 mg or 150 mg twice daily) in patients with AF and at least one additional stroke risk factor (previous stroke or TIA, left ventricular ejection fraction <40%, New York Heart Association heart failure classification of II or higher, age ??JPY75 years, or age 65–74 years plus diabetes mellitus, hypertension, or coronary artery disease).
Patients with stroke within 14 days or those with severe stroke within 6 months, increased bleeding risk, a creatinine clearance (CrCl) <30 mL/min, or active liver disease were excluded.(58) Target international normalized ratio (INR) for warfarin was 2.0 to 3.0. The primary outcome was stroke or systemic embolism; secondary outcomes included stroke, systemic embolism, and death. The primary safety outcome was major hemorrhage. A net clinical benefit was defined as an unweighted composite of stroke, systemic embolism, pulmonary embolism, MI, death, or major hemorrhage.(58)
For the primary outcome of stroke or systemic embolism, both dabigatran 110 mg twice daily (1.53% per year) and dabigatran 150 mg twice daily (1.11% per year) were noninferior to warfarin (1.69% per year); dabigatran 150 mg twice daily was also superior to warfarin (RR, 0.66; 95% CI, 0.53–0.82). Compared with warfarin, the risk of hemorrhagic stroke was lower with both dabigatran 110 mg twice daily (RR, 0.31; 95% CI, 0.17–0.56) and dabigatran 150 mg twice daily (RR, 0.26; 95% CI, 0.14–0.49).
For the outcome of net clinical benefit, dabigatran 150 mg twice daily (6.91% per year) was marginally superior to warfarin (7.09% per year; RR, 0.91; 95% CI, 0.82–1.00) but not dabigatran 110 mg twice daily (7.09% per year; RR, 0.98; 95% CI, 0.89–1.08). There was a trend toward lower all-cause mortality with dabigatran 150 mg twice daily (3.64% year) compared with warfarin (4.13% per year; RR, 0.88; 95% CI, 0.80–1.03) but not dabigatran 110 mg twice daily (3.75%; RR, 0.91; 95% CI, 0.80–1.03).(58)
Major bleeding in RE-LY was lower with dabigatran 110 mg twice daily (2.71% per year; RR, 0.80; 95% CI, 0.69–0.93) but similar for dabigatran 150 mg twice daily (3.11% per year; RR, 0.93; 95% CI, 0.81–1.07) compared with warfarin (3.36% per year). (58) The rate of gastrointestinal bleeding was higher with dabigatran 150 mg twice daily (1.51% per year) than with warfarin (1.02% per year) or dabigatran 110 mg twice daily (1.12% per year; P<0.05).(58) Rates of life-threatening and intracranial bleeding, respectively, were higher with warfarin (1.80% and 0.74%) than with either dabigatran 110 mg twice daily (1.22% and 0.23%) or dabigatran 150 mg twice daily (1.45% and 0.30%).
The rate of MI was higher with dabigatran 150 mg twice daily (0.74% per year) than with warfarin (0.53% per year; RR, 1.38; 95% CI, 1.00–1.91).(58) However, a post hoc analysis found that the MI annual rates of 0.82% per year with dabigatran 110 mg twice daily and 0.81% per year with dabigatran 150 mg twice daily compared with 0.64% with warfarin (hazard ratio [HR], 1.29; 95% CI, 0.96–1.75; P=0.09; and HR, 1.27; 95% CI, 0.94–1.71; P=0.12, respectively) were not significantly different.(59)
There are very limited data on safety in patients taking aspirin or other antiplatelet therapy in combination. Measuring the anticoagulant effect of dabigatran is difficult. Activated partial thromboplastin time, endogenous thrombin potential lag time, thrombin time, and ecarin clotting time can be used.(60) Ecarin clotting time is a clinical assay which can be used to measure the thrombin activity and subsequently is affected by thrombin inhibitors.(60)) Activated recombinant factor VIIa or purified factor replacement products have been proposed for reversal of dabigatran.(61)) Emergency dialysis for rapid reversal of the antithrombotic effect has been recommended.(62) However, there are limited data and there is no widespread consensus on the reversal techniques at this point.(63)(64)
Cost-effectiveness of dabigatran as compared to warfarin has been evaluated. In one study dabigatran 150 mg twice daily had increased quality-adjusted life-years (QALYs) (10.84 versus 10.28 QALYs) as compared to warfarin and an incremental cost-effectiveness ratio (ICER) of $45 372 per QALY gained with dabigatran.(65) Cost-effectiveness analyses have major limitations since they don't take into account the real world clinical practice.
Post marketing, a high number of cases of bleeding with dabigatran led to an FDA analysis. It is important to understand the significant factors that could have affected reporting rates, such as the novelty of dabigatran and the coverage of new drugs in the media, which can greatly influence how and when adverse events are reported.(66)(67)
The most recent FDA analysis showed that the bleeding rates associated with dabigatran are not higher than warfarin.(67) Moreover, patients in the dabigatran arm of the RE-LY trial were followed for another two years as part of the Multicenter Extension of Dabigatran Treatment in Patients with Atrial Fibrillation (RELY-ABLE) study. RELY-ABLE was an observational study and not a clinical trial. Rates of stroke, systemic embolism and major bleeding on dabigatran were similar to rates observed during RE-LY. Total mortality of the two dabigatran groups was similar to RE-LY. Therefore, RELY-ABLE shows that dabigatran is safe on continued follow-up to 4.5 years.(68)
Rivaroxaban
Rivaroxaban is a direct factor Xa inhibitor. It has 70% bioavailability, with a serum half-life of 5 to 9 hours. Clearance is both renal (36% unchanged) and fecal (7% unchanged). The Rivaroxaban versus Warfarin in Nonvalvular Atrial Fibrillation (ROCKET AF) Trial was a double-blind noninferiority trial in randomized patients with nonvalvular AF who were at moderate to high risk of stroke to rivaroxaban (20 mg/d) or warfarin.(69) The primary end point was the composite of ischemic and hemorrhagic stroke and systemic embolism.
In the rivaroxaban group 1.7% of subjects per year reached the primary end point as compared to 2.2% per year in the warfarin group (HR, 0.79; 95% CI, 0.66–0.96; P<0.001 for noninferiority). The primary safety end point was a composite of major and nonmajor clinically relevant bleeding. Primary safety end point occurred in 14.9% of patients per year in the rivaroxaban group and 14.5% in the warfarin group (HR, 1.03; 95% CI, 0.96–1.11; P=0.44). Lower rates of intracranial hemorrhage (0.5% versus 0.7%, P=0.02) and fatal bleeding (0.2% versus 0.5%, P=0.003) occurred in the rivaroxaban group than in the warfarin group.
The most recent FDA analysis showed that the bleeding rates associated with dabigatran are not higher than warfarin.
J-ROCKET AF (Japanese Rivaroxaban Once daily oral direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) was a prospective, randomized, double-blind phase 3 study in Japanese subjects with AF.(70) This study evaluated the safety of rivaroxaban 15 mg once daily (10 mg daily in patients with moderate renal impairment) versus dose-adjusted warfarin. The primary safety end point in J-ROCKET was the time to first major or nonmajor clinically relevant bleeding event in both the rivaroxaban and warfarin arms. There were 11 versus 22 bleeding events in the rivaroxaban and warfarin arms, respectively (1.26 versus 2.61 events per 100 patients per year; HR, 0.48; 95% CI, 0.23–1.00).
The effect of rivaroxaban can be measured through prothrombin time and endogenous thrombin potential. Prothrombin complex concentrate has been reported to reverse the effect of rivaroxaban.(63) Caution is advised regarding these reversal strategies as their clinical efficacy hasn't been adequately evaluated. Rivaroxaban has been shown to be cost effective as compared to warfarin based on quality-adjusted life-years (QALYs) analysis.(71)(72) Postmarketing surveillance data for rivaroxaban is not yet available.
Apixaban
Apixaban is a direct and competitive factor Xa inhibitor. It has 50% bioavailability. It has a short half-life of 8 to 15 hours. Clearance is both renal (25% unchanged) and fecal (50% unchanged).
The Apixaban Versus Acetylsalicylic Acid to Prevent Strokes in Atrial Fibrillation Patients Who Have Failed or Are Unsuitable for Vitamin K Antagonist Treatment (AVERROES) trial was a randomized, double-blind trial comparing the efficacy and safety of apixaban to aspirin in patients with nonvalvular AF who were unsuitable for vitamin K antagonist therapy primarily on the basis of physician judgment or patient preference.(73)
The dose tested was 5 mg twice daily (94%) or 2.5 mg twice daily (6%). The dose of aspirin was 81 mg (64%), 162 mg (27%), 243 mg (2%), or 324 mg (7%) at the discretion of the investigator. The study was terminated when an interim analysis found that apixaban was superior to aspirin for prevention of stroke or systemic embolism (1.6% per year versus 3.7% per year; HR, 0.45; 95% CI, 0.32–0.62) with a similar rate of major bleeding (1.4% per year versus 1.2% per year; HR, 1.13; 95% CI, 0.74–1.75).
Apixaban is a direct and competitive factor Xa inhibitor.
Apixaban was superior to aspirin in preventing a disabling or fatal stroke (1% per year versus 2.3% per year; HR, 0.43; 95% CI 0.28–0.65). The net clinical benefit, a composite outcome of stroke, systemic embolism, MI, death of a vascular cause or major bleeding, supported apixaban as being superior to aspirin (5.3% per year versus 7.2% per year; HR, 0.74; 95% CI, 0.6–0.9). (73)
The ARISTOTLE trial was a phase 3 randomized trial comparing apixaban to warfarin for the prevention of stroke (ischemic or hemorrhagic) or systemic embolization among patients with AF or atrial flutter.(74) The doses tested were 5 mg twice daily as well as 2.5-mg twice daily. Warfarin dose was adjusted to achieve a therapeutic INR of 2.0 to 3.0. Additionally, patients in both groups were permitted to receive up to 162 mg of aspirin daily if clinically indicated.
In the apixaban group 1.27% of the patients experienced the primary outcome of stroke or systemic embolization compared with 1.60% of warfarin group (HR, 0.79; 95% CI, 0.66–0.95). Both noninferiority (P<0.001) and superiority (P=0.01) of apixaban were demonstrated.(74) There was significant reduction in hemorrhagic stroke (49% reduction) compared with ischemic or uncertain types of stroke (8% reduction). Secondary end points of death (3.52% versus 3.94%; HR, 0.89; 95% CI, 0.80–0.99; P=0.047) and major bleeding (2.13% versus 3.09%; HR, 0.69; 95% CI, 0.60–0.80; P<0.001) favored apixaban.(74) Quality-adjusted life-Years (QALYs) analyses have shown apixaban to be cost effective.(75)(76)(77)
Combinations of the Oral Anticoagulants
The safety and efficacy of combining dabigatran, rivaroxaban, or apixaban with an antiplatelet agent have not been established.(54)
Comparison of New Oral Anticoagulants
There is no direct comparison between the anticoagulants so caution is advised when attempting an indirect comparison. There are, however, some features which distinguish these new oral anticoagulants from each other.
The rate of myocardial infarction appears to be slightly higher with dabigatran than with warfarin.(59)(78) It is likely that dabigatran is less effective than warfarin for prevention of myocardial infarction.(79) Moreover, gastrointestinal bleeding was higher with both dabigatran and rivaroxaban than with warfarin.(58)(69) Apixaban use was not associated with higher gastrointestinal bleeding as compared to warfarin.(74) Dabigatran (150 mg twice daily) reduced both the rates of hemorrhagic and ischemic stroke compared with warfarin.(58) Apixaban, in addition to reduction of hemorrhagic and ischemic stroke also reduced major systemic bleeding.(74)
Patients who are noncompliant with warfarin should not be switched to the new agents because missed doses of these short-acting anticoagulants have the potential to be more detrimental than missed doses of warfarin, which has a half-life of several days.(80) Patients who prefer once-daily drugs, or are poorly compliant with twice-daily dosing regimens, can be prescribed rivaroxaban. Patients with hepatic dysfunction should not be given the new anticoagulants because all three agents undergo some degree of hepatic metabolism.
Patients with a creatinine clearance below 30 ml/min also are poor candidates for the new oral anticoagulants.
|
Clinical Vignette Three Mrs. E is a 69-year-old woman with longstanding type II DM and a prior stroke. She has a history of CHF and prior unstable angina (USA) treated with aspirin (ASA). Her kidney function has been in decline — her calculated creatinine clearance is 28 ml/min. She has a history of a bleeding peptic ulcer medically treated in the past. Should she be anticoagulated and, if so, with what? |
|
Clinical Comments This patient is slightly complicated. She is high-risk because of high CHADS2. Moreover, she has chronic kidney disease. Most guidelines advise caution in prescribing newer oral anticoagulants in patients with CrCl<30 ml/min, although they are not absolutely contraindicated. She also has a history of GI bleed, which is a relative contraindication for dabigatran. In her case, the best options are either warfarin or apixaban. |
Generally, in patients with a creatinine clearance of 30-40 ml/min, rivaroxaban or apixaban are better than dabigatran because the degree of renal excretion for them is less than that for dabigatran. Patients with a history of gastrointestinal bleeding reports may do better with apixaban than with dabigatran. A patient with a recent myocardial infarction should be prescribed rivaroxaban or apixaban. Dabigatran may be chosen for patients who suffer an ischemic stroke while on warfarin.(80)
Patients who prefer once-daily drugs can be prescribed rivaroxaban.
Although we have various options for oral anticoagulation available, there is a significant number of patients who have high bleeding risk and should not be anticoagulated. Such patients might benefit from surgical approaches aimed at minimizing the risk of thromboembolism. The procedures specifically target the left atrial appendage, which is considered to be hiding place for thrombus associated with AF, as discussed above.
Left Atrial Appendage (LAA) Closure
Surgical exclusion of LAA has been used for many years to prevent thromboembolism. LAA Occlusion Study (LAAOS) showed benefit in patients undergoing elective coronary artery bypass graft surgery.
The invasive nature of surgical LAA closure, however, limited its use as an adjunctive procedure in patients undergoing mitral valve surgery. A percutaneous approach to close the LAA by implantation of a mechanical device transseptally represents a less invasive method with promising results.(81)
The available LAA closure devices showed efficacy in stroke and cardiovascular death prevention non-inferior to OAC but at a cost of a 5-10% rate of serious periprocedural events. Long-term follow-up data are needed before it can be recommended as the standard of care.(82)
Summary
The new oral anticoagulants represent a major advancement in stroke prevention in patients with atrial fibrillation. There are few points to remember at the end of this Cyberounds®:
- Valvular atrial fibrillation patients should only be prescribed warfarin at this point.
- Newer oral anticoagulants have shown similar or better stroke prevention efficacy in recent clinical trials.
- Newer oral anticoagulants don't require regular laboratory monitoring.
- Newer oral anticoagulants have lower risk of intracranial bleeding as compared to warfarin.
- Newer anticoagulants should be used cautiously in elderly patients as well as those with chronic renal failure.
- Long term data will be available in a few years on all of the newer anticoagulants.
- Antidotes to these medications is currently not available and is being developed.
- Patients who are too high-risk for any anticoagulant therapy can benefit from LAA closure.