Course Authors
Adrian Dubock, Ph.D.
Dr. Dubock is a member of the Advisory Board of the Freiburg Institute of Advanced Studies, School of Life Sciences and School of Soft Matter Research, Albert-Ludwigs-UniversitA?t, Freiburg, Germany. Formerly a senior executive of Syngenta responsible for Mergers, Acquisitions, Ventures and Intellectual Property Licensing, he is currently the Executive Secretary of the Golden Rice Humanitarian Board, and also Golden Rice Project Manager.
Dr. Dubock reports no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff, and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe how a genetically modified crop differs from a conventionally bred crop
Describe the benefits of biofortification compared to fortification or supplementation as exemplified by Golden Rice
Discuss the scientific evidence for more or less caution with respect to genetically modified crops.
Author’s Introductory Note:
I shall use the example of Golden Rice to illustrate the issues with genetically modified (GM) crops, and as this is the first purposefully created biofortified food designed to combat a severe nutritional deficiency I think it will have some resonance for you. The issues raised are, however, of relevance to all GM food crops.
It has been said that: “the anti-side of the GMO controversy tends to be more polemic than scientific.” Nevertheless I have tried to provide a lot of references.
The nutritional trait in Golden Rice causes this rice to produce and retain the pro-vitamin A carotenoid beta-carotene in the normally white part of rice eaten by man.
I started working with the scientific creators of Golden Rice, following publication of their results in Science in 2000. I have worked closely with them ever since, from within the private sector, as well as from within the public sector. Neither of the inventors who donated their technology for the benefit of the resource poor in developing countries in a cashless, royalty free transaction, nor myself, nor any other individual or organization associated with the Golden Rice project has any financial interest in its success.
We have all been and are trying to bring the inventors’ donation of the technology in Golden Rice, free of charge to rice-consuming populations, as an additional intervention for the alleviation of the morbidity and mortality associated with vitamin A deficiency.
The Golden Rice nutritional trait is to be provided free of cost for breeding into local rice varieties. National rice breeding systems in developing countries are key partners for the eventual benefit of resource poor farmers and communities. No one pays any sort of fee. Farmers will be free to grow, harvest, store, consume and locally sell Golden Rice. Consumers will cook and consume it just as for white rice. The technology is in the seed and is stable between generations, and Golden Rice is therefore wholly sustainable. The project is a not-for-profit humanitarian vision-seeking fulfillment.
For more information on the project please refer to www.goldenrice.org or contact me at contact@goldenrice.org .
The Problem

Figure 1. Global Prevalence of Vitamin A Deficiency.
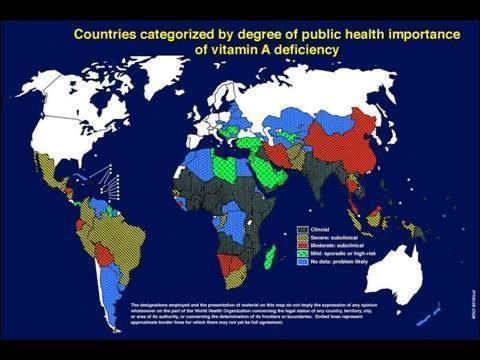
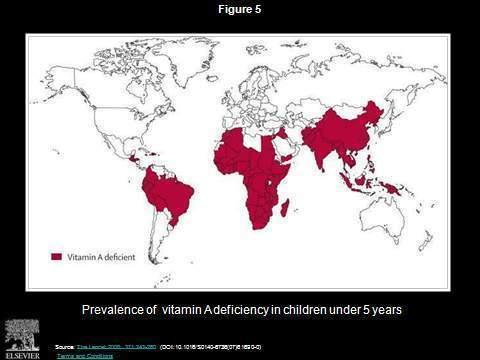

Animals, including man, make vitamin A from carotenoid sources obtained from colored plant food sources, fruit, vegetables and leaves. Animal products, such as milk, eggs, and meat or fish, also contain vitamin A. It can be seen from Figure 1 that the occurrence of vitamin A deficiency is widespread.(1) It is particularly associated with poverty and related dietary insufficiency.
There is a high correlation between the severe occurrence of vitamin A deficiency areas and where rice is grown, especially in Asia and India. Most rice is consumed close to where it is grown: it is usually the staple in those areas.
Rice is the largest provider of carbohydrate to the global population. About 50%, 3.5 billion people, obtain most of their carbohydrate from rice every day. In rice-consuming societies, even young children commonly consume 300 g (dry weight) of cooked rice daily. Adults and children in such societies, especially if they are poor, often eat little else – rice accounts for approximately 90% of their total food consumption. The poor may spend more than 70% of their limited income on food. Price rises make food sources of protein or essential micronutrients essentially unaffordable.(2) Unfortunately, white rice contains little more than carbohydrate, together with tiny amounts of protein and some essential micronutrients such as iron and zinc. But of all the more than 20,000 varieties of rice including colored rices (before Golden Rice was created), none contains any source of pro-vitamin A carotenoid in the part which is eaten, the endosperm.
Chronic consumption of diets that are low in vitamin A content will result in vitamin A deficiency (VAD).
What is the impact of vitamin A deficiency?
Vitamin A is essential for the promotion of general growth, maintenance of visual function, regulation of differentiation of epithelial tissues, embryonic development and immune system function. Additionally, vitamin A has a role in the prevention of morbidity and mortality from infectious diseases in children.(3) Vitamin A deficiency is the leading cause of childhood blindness.(4)

Figure 2. Xerophthalmia.
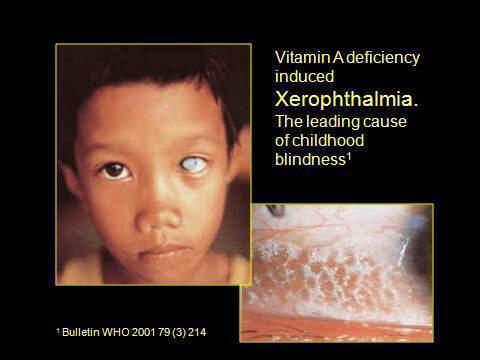

Table 1. Vitamin A Deficiency Disorders.
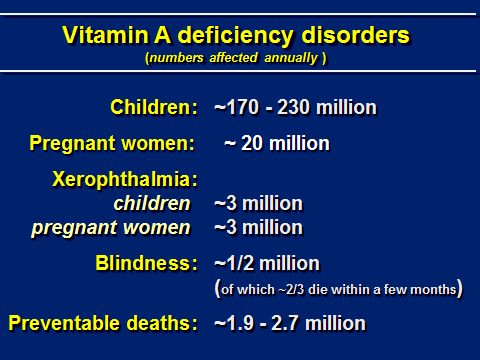
Source: adapted from A. Sommer, pers. comm., incorporating data from Tang et al 2012

Many people are affected by wholly preventable Vitamin A deficiency.(5)(6)(7)(8) Indeed, every single day, year in and year out, vitamin A deficiency kills:
- The same number of people who died from the 2011 Fukushima tsunami.
- Twice as many people as died in the 9/11 Twin Towers attack.
- Twice the number killed in the Pearl Harbor attack which brought the U.S.A. into the Second World War.
The comparison with important disease devastations affecting especially developing countries is also stark,(9)(10)(11)(12)(13)(14) as shown in Table 2.

Table 2. Global Mortality By Disease.
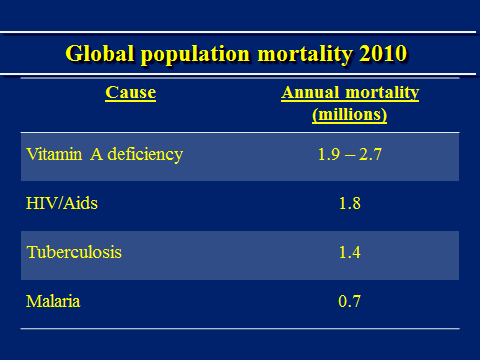

The annual mortality data shown for HIV/ AIDS, TB and malaria are 2010 data. The range for vitamin A deficiency mortality is from the same sources, including WHO and the UN, as in Table 1, and also relate to 2010.
Amelioration of Vitamin A Deficiency
Food-based programs designed to increase the availability of foods rich in provitamin A carotenoids and to promote their consumption have been suggested as realistic and sustainable alternatives to overcome VAD globally.
Further, food fortification, such as fortification of cooking oil, sugar, etc., with vitamin A, is also considered an efficient way to prevent and control vitamin A deficiency.
Since the early 1990s Vitamin A capsules have been administered periodically to “at risk” populations to prevent clinical VAD. Their introduction was controversial. In 1992 the UN International conference on Nutrition concluded: “locally available food-based strategies are the first priority. Supplementation is an interim measure.”(15)
Chemically synthesized vitamin A (~500 million vitamin A capsules per year) have a total cost, including distribution, of around $I.0 billion annually.(16) Currently, most of this cost is paid by U.S. and Canadian taxpayers through their governments’ international aid agencies.A recent review and meta-analysis(17) concluded: “Vitamin A supplementation is associated with large reductions in mortality, morbidity, and vision problems in a range of settings, and these results cannot be explained by bias. Further placebo controlled trials of vitamin A supplementation in children between 6 and 59 months of age are not required. However, there is a need for further studies comparing different doses and delivery mechanisms (for example, fortification). Until other sources are available, vitamin A supplements should be given to all children at risk of deficiency, particularly in low and middle income countries.”
Beta-carotene is an important source of vitamin A for humans(18) and is ubiquitous in plants, and a balanced diet. However, the efficacy of carotenoid-rich plant foods in the prevention of VAD has been shown to be lower than previously expected: the vitamin A equivalency of provitamin A carotenoid-rich foods varies from 2 μg beta-carotene (β-C)-to-1 μg retinol (for diets containing pure β-C in oil) to 27 μg ß-C-to-1 μg retinol (for ß-C in certain leafy vegetables).(19)
Unlike Vitamin A itself, beta-carotene at the physiological levels found in food crops, including Golden Rice, are Generally Considered as Safe (GRAS),(20) even when consumed at elevated levels for years.(21)(22)(23)(24) The human body does not absorb or convert (to vitamin A) what it does not need. [There is evidence (from beta-carotene, selenium and iron) that at very significantly higher pharmacological doses of these essential micronutrients, repeat consumption may not be benign.(25)
In humans (adults and especially children) the bioconversion ratio of the β-C from Golden Rice to retinol, the most prevalent circulating form of Vitamin A, is close to the theoretically most efficient as from pure β-C in oil. This research conducted with adults and children in the U.S.A. and China suggests that: ”Golden Rice may be as useful as a source of vitamin A as preformed vitamin A from vitamin A capsules, or eggs and milk.”(26)
The Recommended Daily Allowance (RDA) of Vitamin A determined for the adult population of the U.S.A. includes sufficient intake to maintain three months of liver stores. Maintenance of liver stores is not required to combat morbidity and mortality arising from vitamin A deficiency. When taken together with other sources of dietary pro-vitamin A usually present even in impoverished diets in developing countries, nutritionists consider that 40% of the Estimated Average Requirement (EAR) of vitamin A daily is sufficient to combat mortality and morbidity from vitamin A deficiency. (RDA is statistically derived from the lower EAR figure which has been directly determined).
To deliver 40% of the EAR, taking into account all the relevant variables for Golden Rice involved in growth, storage, cooking, consumption and bioconversion of the beta-carotene, requires only about 40 g of dry rice to be consumed, as cooked rice, daily. Forty grams is about the amount of rice contained in the Petri dish in Figure 3 below. (The rice plants in the background are Golden Rice plants.)

Figure 3. 40 Grams Daily of Golden Rice: Saving Lives and Sight.

Image courtesy of Golden Rice Humanitarian Board www.goldenrice.org

Ex ante economic impact analyses of Golden Rice forecasts a significant impact on the wealth of Asian countries: conservative Golden Rice consumption could add from 4 to 18 billion dollars annually to Asian GDP.(27)(28) By enhancing the effectiveness of their immune systems, the well being of the poorest individuals in society would be significantly improved by consumption of Golden Rice, and agricultural productivity would increase markedly.
A World Bank study (1994) assigned a present value of US$45 to each annual case of iron deficiency averted and US$96 for each annual case of vitamin A deficiency averted for preschool children.(29)
Golden Rice ex ante studies(30) assumed a 12:1 bioconversion of beta-carotene to vitamin A, and suggested that Golden Rice would cost US$3.00 per person, adult or child, to prevent mortality and morbidity caused by vitamin A deficiency. All of the $3.00 costs are incurred in the development of the Golden Rice. There are no extra costs of rice growing or preparation associated with Golden Rice compared to white rice. Recent data show that in Chinese children the bioconversion (at 2.3:1) of the beta-carotene in Golden Rice to vitamin A is not significantly different from the (2.0:1) bioconversion of beta-carotene in oil to vitamin A, and better than from spinach in the same research (7.0:1.0).(31) These data suggest that the full (development) cost of Golden Rice will be closer to $0.50 per person. Similar ex ante cost-benefits have been calculated for putative multi-biofortified rice in China.(32)
These estimates derive from the knowledge that once a biofortified crop is created and is stable, there is no additional cost associated with its cultivation and use: all the costs of a biofortified crop are in the initial development, making the initial seed available, and related education and social marketing programs. This is different from food fortification with micronutrients, or supplementation of minerals and vitamins, where significant manufacturing and distribution costs are implied, which impacts availability for the most needy.(33)
Nevertheless, even using these significant costs of fortification or supplementation, a regular meeting of Nobel laureate economists – the Copenhagen Consensus – has consistently ranked in each of its three meetings over the past years, micronutrient provision as the highest or second highest priority for cost-effective solutions to the thirty most pressing problems of mankind. Indeed, addressing micronutrient malnutrition has usually been listed as two or three of the top five or six priorities on each occasion (Copenhagen Consensus 2012, 2008; 2004(34)).
By the time it is in use, a biofortified crop such as Golden Rice – whatever technology is employed to create it – should cost no more to grow than the variety from which it is derived.
If the technology is provided free of charge, as in the case of Golden Rice, then the consumer will truly benefit nutritionally. By carrying the nutritional trait in agronomically excellent germplasm varieties of the crop which grow well, and where the local people enjoy the cooking and eating qualities of the biofortified crop, adoption should not be very challenging.

Table 3. Copenhagen Consensus Priorities.
|
2012 |
|
Challenge | Solution |
|---|---|---|---|
|
|
1 | Hunger & Education | Bundled micronutrient interventions to fight hunger & improve education |
| 2 | Infectious Disease | Expand subsidy for malaria combination therapy | |
| 3 | Infectious Disease | Expand childhood immunisation coverage | |
| 4 | Infectious Disease | Deworming of schoolchildren to improve education & health | |
| 5 | Infectious Disease | Expand tuberculosis treatment | |
| 6 | Hunger, Biodiversity & Cl. Change | R&D for yield enhancement to decrease hunger, fight biodiversity destruction, lessen climate change effects | |
|
2008 |
|
||
|
|
1 | Malnutrition | Micronutrient supplements –children (vitamin A & Zinc) |
| 2 | Trade | The Doha Development Agenda | |
| 3 | Malnutrition | Micronutrient fortification (iron & salt iodization) | |
| 4 | Diseases | Expanded immunization coverage for children | |
| 5 | Malnutrition | Biofortification | |
|
2004 |
|
||
|
|
1 | Diseases | Control HIV/AIDS |
| 2 | Malnutrition | Providing micronutrients | |
| 3 | Subsidies & Trade Barriers | Trade Liberalization | |
| 4 | Diseases | Control of Malaria | |
| 4 | Malnutrition | Development of new agricultural technologies | |

In most nutritionally disadvantaged and often impoverished populations, individuals are very keen to improve their and their family’s nutritional status, especially if there is no associated cost increase.
VAD - Problem Solved?
Well, not yet. Golden Rice exists. It has existed since 1999, initially with a lower content of beta-carotene. We know now – but nobody could have definitively known at the time because the human bioconversion efficiency had not yet been determined – that these levels would have probably made a significant impact on the reduction of VAD, for they deliver approximately 40% EAR in 40 g consumption daily of Golden Rice, well within adult consumption patterns in populations where rice is the staple food.
In 2000, in return for formal undertakings to the inventors being included in licenses with Syngenta, the inventors licensed to the company the commercial applications of the technology in a costless, royalty free transaction. The initial form of Golden Rice worked with one or two genes introduced from a daffodil and also one from a common soil bacterium. (The soil bacterium gene was chosen by Peter Beyer, as bacteria use one gene to do what plants need four genes to do, and transforming the rice plant with two genes is easier than transforming the rice plant with five genes.)
The inventors retained rights to carefully but generously defined humanitarian uses, necessary for fulfillment of their altruistic vision. Syngenta obligations entered into included supporting regulatory submissions and donating technology improvements to the inventors for the humanitarian uses. In 2004 and 2005, Syngenta scientists, in pursuit of commercial goals, modified the genetic engineering approach, to include a maize gene, the soil bacterium gene and no daffodil genes. This led to higher levels of expressed carotenoids, and a higher proportion of the important carotenoid β-carotene. Pursuant to the earlier agreement this was supplied for humanitarian purposes.(34)
The (improved) trait has been and is being introgressed into major varieties of Asian rices to match the agronomic and cultural preferences of the geographies and populations most affected by VAD. The final genetic type of Golden Rice, to be introduced into all rice varieties everywhere, was selected in early 2009. At the time of writing, it is mid-2012 and Golden Rice is in five countries only, and in those five countries only with public sector research institutions, not growers. As a 2009 editorial in The Lancet stated: “If undernutrition were a disease, such as H1N1, and unprocessed food were a drug or a vaccine, both would have the full attention of the entire international community.”(35) It is expected that Golden Rice will be cleared for use by farmers and consumers in one country during 2013.
Normally, a variation on an established rice variety, which offers advantages over predecessor varieties, can be adopted by growers and then, of course, by consumers of the rice very quickly. A rice variety known as ‘Swarna-Sub1’, conventionally bred in India to be able to survive the normally fatal period of submergence in flood water in excess of 14 days , was first grown in open fields in 2009. Two years later it was already the fifth most popular rice variety planted in India,(36) and in its third year the third most popular(37) after ‘Swarna (without submergence tolerance)’ as the number two variety.
In contrast, the improved Golden Rice, created using genetic engineering techniques, was first grown experimentally in an open field at Louisiana State University in 2004. Yet no developing country growers have it anywhere, and are unlikely to have it freely until 2013, almost 10 years after the first field growth.
Why the delay with Golden Rice?
In 1996, the world’s first food produced by precise introduction of specific genes was put on sale in the UK by Zeneca (now Syngenta). The canned tomato paste was clearly labeled as derived from ‘genetically modified tomatoes’ created to remain firmer for longer. This modification allowed for cheaper transport to the processing plant, required less energy to manufacture the paste and less energy to reduce the cooking sauces when used in the home.
The genetically altered tomato paste was offered for sale alongside conventionally produced tomato paste on the shelves of two UK supermarket chains. Clear information about the technology involved to initially produce the seed was prominently displayed on the packaging and at the point of sale.

Figure 4. Genetically Modified Tomato Paste.


These cans of genetically modified tomato paste, which were slightly cheaper per can, outsold the conventionally produced tomato paste by 2:1. European consumers were not antagonistic to ‘genetically modified’ foods.
In 1997 Monsanto tried to introduce animal feed – soya and maize meal – made from U.S. grown genetically modified crops into Europe. Europe had recently had a number of food safety scares, including an outbreak of bovine encephalitis ‘Mad Cow Disease’. The company assumed that the European population’s initially negative reaction to the genetic modification technology would gain reassurance from being told that the U.S. government judged the products safe, and Monsanto tried to use this argument. This misjudgment of the cultural differences between North American and European attitudes with respect to trust in governments inflamed the suspicion of the European public’s concern, and public reaction became hysterical. “Frankenfoods” became an almost daily headline in the UK tabloid press(38)(39) and with strong echoes in continental Europe particularly.
The Zeneca tomato paste on sale in UK was being produced in and shipped from California. This was uneconomic on a sustained basis. Yet with the European furor about genetically modified food, it was clear that permission to grow the tomatoes in Europe – required only because they had been produced with the technique of genetic modification – could not be guaranteed in the short term. Production ceased and stocks were sold out.
Into this deeply suspicious environment the announcement of Golden Rice’s creation occurred,(40) instantly provoking vehement activist opposition against the project and its aspirations. A variety of reasons were given(41)(42)(43) and, in a pattern which was to become established, a polarized debate for and against became, and remains, a favorite academic topic for biology textbooks, classrooms and political discourse.(44)(45)(46)(47)
Greenpeace put out an early press release claiming an adult woman would have to eat between 9 kg and 18 kg of cooked Golden Rice daily to gain any advantage from the beta-carotene contained in it.(48) (Interestingly, this increasingly obvious egregious lie has been removed from their website.) The Greenpeace claim was echoed by opponents of Golden Rice immediately. We now know that a few hundreds of grams daily, even of the Beyer and Potrykus prototype Golden Rice, could have saved lives and prevented blindness.
Starting before publication of the work of Potrykus’ and Beyer’s teams, hints of the politics of opposition were apparent. A manuscript describing the ground-breaking research and results from turning on a metabolic pathway for carotenoid biosynthesis with two or three genes – at the time all commercial genetically engineered products only included one new gene – was first offered to and then rejected by Nature, a European-based journal. Eventually, the breakthrough, which came to be known as Golden Rice, was published by Science(49), a U.S.-based journal. The research was subsequently recognized for its importance – the Science paper was ranked as among the most cited plant biotechnology papers from 1999-2005(50) and the two professors were recognized in 2006(51) as the most influential industrial, agricultural and environmental biotechnologists of the last decade.

Figure 5. Golden Rice Media Attention.


A planned 45-minute personal audience with Pope John Paul II for one of the inventors, Professor Ingo Potrykus and his wife, was changed at the last moment to a public audience lasting a few seconds, following internal Vatican lobbying reportedly by Catholic cardinal(s) from a country politically opposed to genetic engineering. Golden Rice was featured on the 31 July 2000 cover of the U.S. edition of Time magazine(52) with the headline “This rice could save a million kids a year, but... ”, and also featured in Asian editions (with a different Golden Rice cover picture.) Golden Rice was not, however, even reported in the European edition of Time.
Other activists claimed that to support Golden Rice would divert attention from efforts to support the elimination of poverty, and that existing interventions to combating vitamin A deficiency would be undermined.(53)
Greenpeace was a leading opponent, and their opposition continues unabated.(54)
Not everyone was negative but the negatively expressed views far outweighed the positive support for the project. A 2000 cartoon, from Des Moines, Iowa, the center of the U.S. corn belt, nicely summarized the situation:(55)
Humor was tried as an antidote to the negative:

Figure 7. Another Cartoon in Support.


Other cartoons, perhaps, came close to reflecting what was in the minds of some opponents:

Figure 8. Cartoon Against the Program.


After all, when successful, Golden Rice will torpedo most of the arguments used by anti-GMO crop activists. It does not profit multinational companies. Golden Rice is not for the benefit of industrialized nations and their industrialized farmers. For the nutritionally disadvantaged rice-eating populations of developing countries, unlike most genetically engineered crops to date, Golden Rice is expected to offer an easily understood consumer benefit: better nutrition leading to lower mortality and better vision. And free for the nutritional trait.
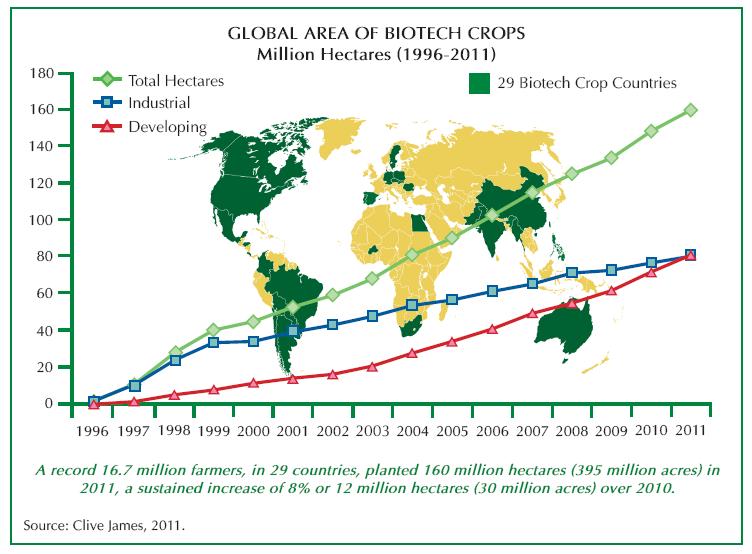
There was, perhaps still is, a belief that Golden Rice was (is) a “Trojan Horse” of the commercial companies to open up a “reluctant market” for genetically modified crops. In reality the agricultural market is not so reluctant (except in much of Europe). By 2011, 16.9 million farmers in 29 countries were growing genetically engineered maize, soya, cotton, canola, potatoes, alfalfa, sugar beets, squash, papaya, tomatoes and sweet peppers. At a percentage compound growth rate in double figures, the global growth of genetically modified crops has not been exceeded by any other agricultural technology in history.(56)

Figure 9. Global Planting of Biotech Crops.

The research of Ewen and Pusztai(57) on potatoes, and Losey et al.(58) on Monarch butterflies were widely regarded, at least in the popular press, as justification for the political opposition. Both groups concluded that there were unacceptable adverse effects of genetic modification. However, in both cases the experimental design did not support the conclusions reached by the authors, nor was it possible for the research results to be replicated by others. The quality of the science in both cases was widely discredited after publication, making any valid use of the results highly questionable.(59)(60)(61)(62) Nevertheless, some very vehement opposition continues to be extremely alarmist.(63)
It is difficult to summarize briefly the arguments of many of those opposed to genetically engineered crops, as the arguments are very wide ranging.(63) However, they include:

Table 4. Arguments Against Genetically Modified Crops.


These issues could in all cases be as easily directed at conventionally bred crops as at genetically engineered crops.(65)(66)
Additionally, some opponents of the technology are concerned – but without evidence – at a more fundamental level that adverse biochemical changes may be induced either by the process of genetic modification itself, or the pathways affected, or that environmental effects may be caused which are irreversible.(67) No substantiated data has been put forward to justify these concerns.(68)(69)
Opposition to Golden Rice has included accusations that its (very minimal!) funding should instead be used to address the elimination of poverty. Another accusation is that biofortifying a staple crop may encourage a limited diet rather than a more ideal dietary diversity. Similarly, it is argued that small gardens and consuming a traditional range of locally available food crops are more acceptable.(70) All of these positions appear to ignore the existing statistics, and related human misery, on the human toll of vitamin A deficiency(71)(72)(73)(74) and that Golden Rice is offered as an additional intervention to the others, not as a replacement for them, in addressing vitamin A deficiency.
For more than a decade Golden Rice has had a pivotal role in the arguments for and against genetic engineering, and all its challenging research, with very little funding, has been carried out against that backdrop. As an example, campaigners for the “Keep Wales GM Free” campaign accused the scientists involved and their institutions, as well as the whole Golden Rice Humanitarian Board, of “crimes against humanity” and transgressing the Nuremburg Code because of the very necessary research involving Chinese children done to establish the bio-conversion efficiency of the beta-carotene in Golden Rice to vitamin A. The campaign ignored the facts that animal models were not suitable, that beta carotene at the levels found in food matrices is safe and that the management of such clinical trials followed accepted international regulation and practice by the clinicians and medical institutions involved! Two independent ethical reviews agreed with us and disagreed with the anti-GM activists.
The activists’ concerns about genetic engineering are curious, given that genetic modification of organisms is the basis of many widely adopted, and widely accepted products. For example, bread, cheese, beer and wine makers all routinely use genetically engineered enzymes as an important part of the production process. Similarly, insulin for the treatment of diabetes is produced using genetically engineered organisms.
The strongest opposition to genetically engineered crops arose in Europe, and is often promulgated by activist organizations based in Europe, some of whom express concern about damaging exports to Europe.
How does genetic modification of crops differ from conventional breeding of crops?
Crop breeding started as soon as man stopped being a ‘hunter gatherer’ and started farming. It is reputed that selection of wheat seeds for improved yield started in Mesopotamia (now Iraq) 10,000 years ago.
Many new crops have been introduced to previously un-exposed human populations over the course of history:(75)

Figure 10. New Crop Introductions Historically.

Breeding of crops has concentrated on yield: a combination for cereal crops of increased carbohydrate production in the face of biotic and abiotic stresses on the plant.

Figure 11. Science and Innovation Drive Crop Yields.
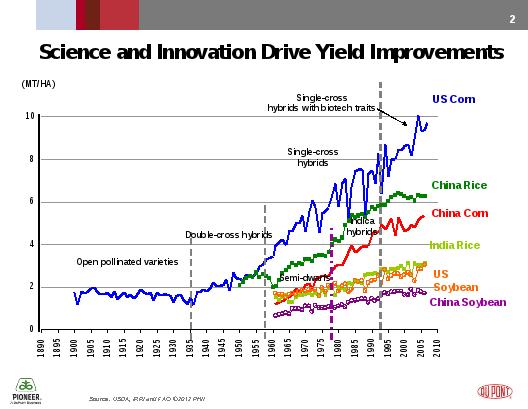

Only very recently, in historical terms, has the importance of micronutrients been ascertained, and even more recently the methodology to measure the content of the micronutrients in the diet has been developed.
For a plant breeder to achieve desired improvements to crop traits, there has to be variation in the amplitude of the trait in individuals of the population. By breeding together (introgressing) such individuals, the random processes of meiosis and mitosis involved generate progeny with F1 vigor from which the direction of the desired improvement can subsequently, by repeated introgression, be selected.
Plant breeders use many systems to stimulate variation(76) in a population of plants:

Figure 12. Inducing Variation for Crop Breeding.
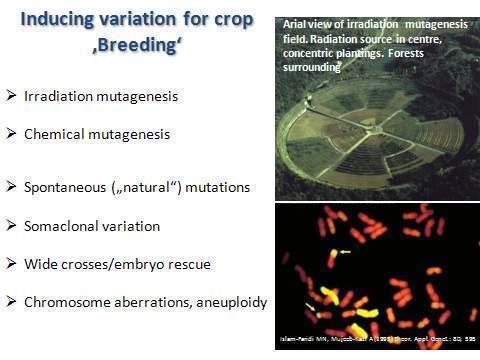
Source: The chromosome rearrangement image is from Islam-Faridi MN, Mujeeb-Kazi A (1995) Theor. Appl. Genet.: 80, 595.

All of the above systems are responsible for random genome alterations in the progeny, from which the breeder selects useful individuals for the next round of breeding. Somaclonal variation, for example, describes the random variation which occurs between clones of a plant grown by culturing cell callus produced by culturing individual cells. The cloned plants are often not identical. Cells can double their chromosome complement; lose genes or even whole chromosomes. When the callus develops into a plant, it may not be genetically or phenotypically identical to its parent.
Chemical and irradiation induced genetic mutations are also commonly used in plant breeding, and have been for decades. There are, for example, more than 16,000 mutant varieties of rice.(77)
Most of these processes of genome alteration are “unnatural.” All however are considered to result in “conventionally bred crops” which can be introduced to human populations, or used as animal feed sources, without exhaustive understanding of what difference the random genome alterations have made to the biochemistry of the plants concerned. In the terminology of the anti-GMO activists, none of these systems results in plants which are “genetically modified.”
In random genome alterations typical of conventional plant breeding, hundreds (sometimes thousands) of genes of the small rice plant genome (of approximately 30,000 genes) are involved. With genetic engineering, in contrast, usually one or two genes and their control elements are introduced to the genome. The position of the insertion of the genes by "conventional" or "engineering" methods is random in that it cannot be controlled precisely. In both cases useful phenotypes are selected by the plant breeder and others discarded. But only in the case of genetic engineering does society’s suspicion of the technology require extensive, expensive and time consuming research to completely understand the molecular structure of the genotype(78) and environmental impact.(79) This requirement is encompassed in a bureaucratic framework of national regulations, currently not aligned between nations, and springing from environmental protection inspired by the UN Convention on Biodiversity(80) and its highly precautionary Cartagena Protocol.(81)
Given that both “conventional breeding” and “genetic modification” approaches result in random genetic modification, it has been suggested that “genetic engineering” is a more appropriate terminology for what the activists call “genetic modification” as in GMOs – Genetically Modified Organisms.
Both “conventionally bred” plants and “genetically engineered” plants can generate traits,(82) which may be discarded or retained by the plant breeders in both cases.


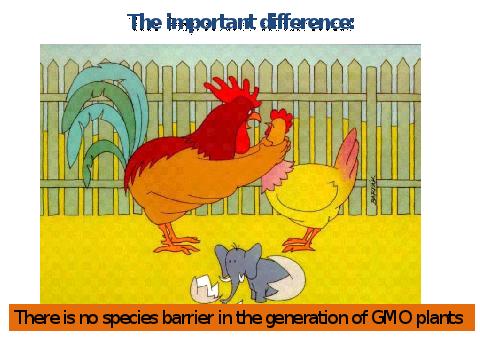
Genetic engineering takes advantage of the 1) evolutionary conformity of genetic control, and 2) gene-process-uniformity of the same or similar biosynthetic pathways and their controlling genes, in different organisms. This conformity enables scientists to introduce new genes across species or ecosystem barriers which would not otherwise allow the production of fertile offspring through biological reproduction. In genetic terminology, we are able to introduce tissue specific expression control from an organism where such control is switched on to one where previously the same gene was not switched on in the same tissue.

Figure 13. Overcoming the Species Barrier.

Examples for both advantages in plant breeding can be found in Golden Rice. The current version of Golden Rice, the “lead transformation event” which is being introgressed into all varieties of rice, is based on two introduced genes. One of them, a gene which already exists in “white rice” varieties, is not - in the “white rice” varieties - active in the rice endosperm. The same gene has been taken from maize, with the component which activates it in maize endosperm, and added to rice so that the gene is then active in rice endosperm ("evolutionary conformity of genetic control"). The second introduced gene into Golden Rice is derived from a common soil bacterium. It is introduced into Golden Rice as bacteria accomplish with one gene, what plants in their carotenoid biosynthetic pathway need four genes to accomplish, and those four genes are not activated in “white rice” endosperm (“gene-process-uniformity”).(83)
When the bacterial gene, and the maize gene, in both cases with their controlling genetic transducers are included in the white rice genome, under endosperm specific expression activation, Golden Rice, producing very useful beta-carotene, is the result:(84)

Figure 14. White Rice and Golden Rice.

Image courtesy of Golden Rice Humanitarian Board. www.goldenrice.org

Why is genetic engineering useful at all?
In some crops there is too little variation to breed from. None of the 20,000 varieties of rice has any carotenoid in the endosperm. In some crops sexually mediated breeding is very difficult.

Figure 15. Traits Difficult to Breed.


Sometimes the trait of interest does not occur “naturally.” But remember that all crop breeding for the past 10,000 years has been through the efforts of man. Whether that is “natural” or “unnatural,” depending on one’s perspective, exploiting modern scientific knowledge to understand the benefit of new traits, and to introduce sources of them, is equally “natural” or “unnatural,” again depending on one’s perspective. And it has been pointed out that nature uses genetic modification,(85) that prehistoric corn is genetically modified,(86) that every traditionally bred modern variety is most intensively genetically modified(87) and that even the term ‘genetically modified organism’ is therefore a misnomer.(88) One could argue that all agriculture is unnatural. But is it clearly necessary to produce the nutritious food we need.
A continent which has been slow to adopt modern agricultural technology is Africa.(89)

Table 6.
Per Capita Food Production By Continent.
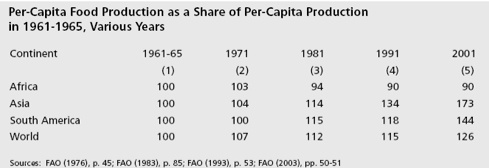
Africa has the lowest use of all the continents of improved seed, and of fertilizer. It is the only continent where agricultural productivity declined over the last 50 years.
One country in the world insists on using old-fashioned agricultural technology, North Korea. Repeatedly it fails to produce enough food and its people starve.(90) Before China adopted technology it was in a similar situation. But now China is investing more than $3 billion in modern plant biotechnology as it seeks food sufficiency for its population.(91)
It is sometimes stated that the world produces enough food, it’s just in the wrong places. This is probably true, as is the fact of huge (~30%) post harvest losses and an unquantified, but also huge in some countries, post-food-preparation loss. However, as a supporting argument for not adopting an agricultural technology, this is as cruel to today’s needy people as stating that working on Golden Rice diverts attention from other approaches to assist the reduction of poverty!
What do the world’s scientific authorities say about GMOs?
Repeatedly, academies of science and other impartial evidence-based institutions, from all parts of the world, have found no evidence to support the contention that the techniques involved in genetic modification are any more hazardous to man or the environment that “conventional plant breeding” (Table 7).

Table 7. Institutional Support for Genetically Modified Crops

© Douglas Southgate and Douglas Graham, 2006
Published August 2006 by International Policy Press on behalf of the Sustainable Development Network

The Food and Agriculture organization of the UN declared: “No verifiable untoward toxic or nutritionally deleterious effects from the consumption of foods derived from genetically modified foods have been discovered anywhere in the world.”(92)
Surprisingly given the pervading European attitudes to GMOs the European Commission of the European Union stated: “The main conclusion to be drawn from the efforts of more than 130 research projects, covering a period of more than 25 years of research and involving more than 500 independent research groups, is that biotechnology, and in particular GMOs, are not per se more risky than e.g. conventional plant breeding technologies.”(93)
Similarly, U.S. institutions(94) have ranked the risks from different sources of variation for plant breeding, and found that genetic mutational breeding – that is, artificially inducing random genetic mutations as described above – which is considered to be a technique of “conventional breeding” (with which anyway there are no practical problems) to be riskier than the techniques used in genetic engineering (Figure 16).

Figure 16. Rank Order of Risks By Plant Breeding Techniques.
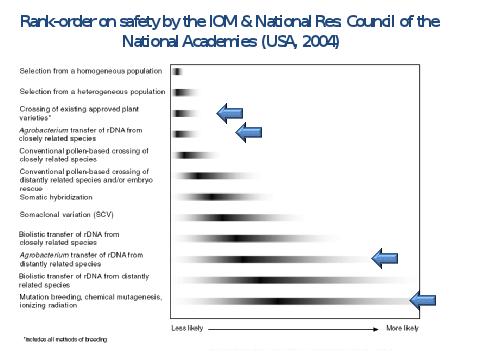
Adapted from: Safety of Genetically Engineered Foods: Approaches to Assessing Unintended Health Effects. 2004. From The National Academies Press, 500 Fifth St, NW, Washington, DC 20001, ISBN 0-309-09209-4.

Following a quarter of a century’s investigation, including sixteen years since genetically modified crops were introduced commercially, and millions of dollars of intensive research looking for adverse effects, not one substantiated environmental or human health adverse effect has been found.(95)(96)(97)
In passing, it may be worth mentioning ‘organic crops.’ The arguments between conventional and “technified” farming and what some see as the latter’s worst element – genetically modified crops – are often conflated and compared with organic farming. The term ‘organic agriculture’ is inaccurate and misleading.(98) Scientific evidence is lacking for any nutritional benefit(99) from ‘organic food’ that justifes the higher costs of production and purchase.(100) I have heard a senior member of the UN’s International Fund for Agricultural Development explain that organic agriculture is environmentally unsustainable because it takes more out of the land that it puts back. Moreover, given that extensive use of animal manures and resulting methane production from the ruminants involved produce significant detrimental effect on global warming, organic farming seems unwise even if there is enough water to grow all the forage crops needed. Normal Borlaug, Nobel Peace Prize winner for his work which led to the ‘Green Revolution,’ has said that organic agriculture could feed about four billion of the world’s population. “Which three billion should die?”(101)
One could say that the lobby for organic food made a strategic error by not embracing the technique of genetic engineering of crops. What could be more “organic” than plants defending themselves against biotic or abiotic stresses, or making themselves more nutritious, as a result of their own genetically controlled processes?
Does opposition to, and suspicion of, GMO crops matter?
There are supportable arguments that intensive agriculture – using all possible means to grow the most food, feed and fiber on the least land area – protects wild land and biodiversity while at the same time producing the necessary quantity and quality of food for mankind with the least labor. Intensive agriculture requires the utilization of technology. The Pontifical Academy of Science, based in the Vatican, has considered the issue of genetically modified crops and found them to be “pro-poor.”(102)(103) Other institutions concerned with ethics have similarly concluded that it is unethical to block the deployment of a technological capability where such technology can reduce poverty, or the effects of poverty. “The moral imperative for making GM crops readily and economically available to developing countries who want them is compelling.”(104)
Yet for complex reasons, many large institutions – from the private, philanthropic and public sectors – remain wary of fully supportive engagement with the technology. It seems they have absorbed as “commonly accepted wisdom” the initially Europe-originated suspicion of the GMO technology,(105) or at least are concerned that they will suffer reputational damage as a result of association with it if activists make a lot of noise.
However, perhaps the tide of public opinion concerning genetically engineered crops is turning. The phrase ‘Frankenstein foods’ first use is credited to a letter in The New York Times on June 16 1992. Subsequently the British newspaper, The Daily Mail, used the same phrase in a headline in February 1998(106) and thereafter extensively used the shortened form ‘Frankenfoods’.In May 2012 the G8 meeting at Camp David hosted by President Obama discussed new efforts to energize agriculture in Africa. President Obama recognized that to improve agriculture and nutrition is “a moral imperative, it's an economic imperative, and it is a security imperative.” The head of USAID added: “You cannot have stability and security as long as regions and countries and communities are deeply food-insecure.”(107)
In the same month government researchers at a UK plant research station were involved with opponents of their genetically modified wheat field trials. The online version of the UK newspaper, The Daily Mail, which had run a vitriolic campaign for so long in the UK against genetically engineered crops starting in 1998, in June 2012 decried the activities of the anti-GMO protesters almost as vehemently as it had 14 years earlier protested the technology. The 2012 article: “Frankenfoods - the lesser of two evils?... green opposition to GM is starting to look outdated, hysterical and profoundly anti-human.”(108)
In 2009 a Vatican meeting reported: “The magnitude of the challenges facing the world’s poor and undernourished must be addressed as a matter of urgency. Every year nutritional deficiencies cause preventable illness and death. The recent rise in food prices throughout the world has revealed the vulnerability of the poor to competition for resources. In this context, forgone benefits are lost forever.”(109)
Unreasonable opposition has blocked for too long genetic engineering applications in crop science, which can be another very useful tool in the plant breeder’s toolbox, yielding beneficial plant traits not achievable by other means. From the start, Greenpeace in all countries has opposed the technology. The founder of Greenpeace’s comments on the activists is interesting:
“Why then do anti-GM forces continue to make a concerted effort to drown out good news story with misinformation and propaganda? I believe it is because they do not care about human welfare or the environment for that matter, but are determined to strike a blow against the globalisation of agriculture, multinational corporations, and capitalism in general. This campaign works for them because they are able to scare a large segment of the public who do not have an understanding of this relatively new science, which is both invisible and complicated. Despite the fact that there is not one iota of truth in their campaign of fear, they succeed with many people who are afraid of the unknown.
There is also a growing trend among environmental activists to take on campaigns they will never win in the foreseeable future. They will never stop the growth of GM technology; they will never stop nuclear energy or fossil fuel energy, they will never stop the sustainable management of forests for timber production; and they will never stop salmon aquaculture. This creates an opportunity for an endless campaign of propaganda, supporting an endless fundraising campaign to support even more propaganda. As a political strategy it is quite brilliant, except that they didn’t actually devise it themselves, it just happened that way. It happened that way because the campaigns they won are now over, and as they gradually abandoned science and logic in favour of zero-tolerance policies, they inevitably ended up with unwinnable campaigns. Unfortunately we will have to put up with these campaigns for a long, long time.”(110)
With respect to Golden Rice’s potential impact in reducing mortality and morbidity resulting from vitamin A deficiency, one can speculate about the impact of different societal acceptance of GMO’s on achievement of the Millennium Development Goals(111) #4, #5 and probably #6 (also due to the immune response suppression effect of vitamin A deficiency):

Figure 17. Millennium Development Goals.


Without some accelerated improvement, target #5, reduction of under 5- years child mortality, will not be reached by 2015 (Figure 18).

Figure 18. Actual Progress Towards Reduction In Under 5-Year Mortality.
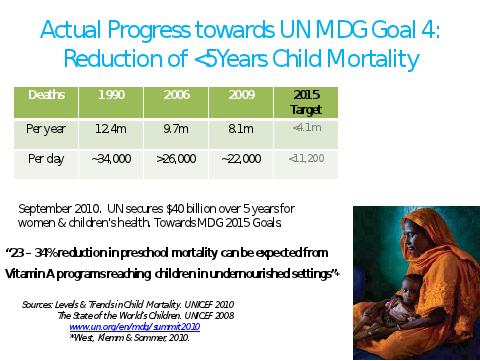

We can only wonder what would have been the effect on the achievement of this target if the adoption of established rice varieties containing the Golden Rice biofortified trait had been achieved as quickly as is normal for conventional varieties.
It seems that societal attitudes to GMOs, manifest in suspicion of the technology and related training of governments in GMO risk assessment, will have prevented achievement of this UN Millennium Development Goal target. The problem is an overly rigorous regulatory system which, against all scientific reason, forces a transgenic event to be selected and characterized in the first place. When irradiation or chemical mutagenesis is used to induce variation from which to select phenotypes for breeding, no one knows the molecular structure. And no one is forced by regulation to characterize it before use or to select only genotypes defined by specific molecular characteristics. When 5000+ people die of vitamin A deficiency every day despite current interventions, unwarranted delay is unreasonable.
It is for this delay that Patrick Moore, accused Greenpeace, the organization he founded, of ‘crimes against humanity’ for their opposition to Golden Rice.(112)
It may not be as easy to propose gross societal benefits from agronomic traits introduced into crops by genetic modification in such a stark manner as is possible for potential health benefits. But such benefits have been described by the UN’s Food and Agriculture Organization, for example from the introduction of genes imparting insect resistance into cotton in place of chemical insecticides (Table 8).

Table 8. Benefits from GMO Crops.
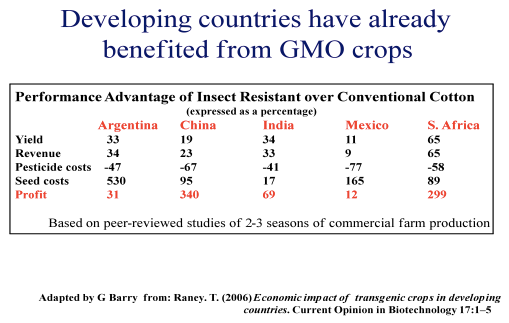

For many other crops, millions of farmers globally, who are free to plant any seed they desire, have chosen to adopt this technology for its agronomic benefits.(113) In so doing, they are also expressing their support for genetic engineering.
Conclusion
Given that genetic engineering is widely used in processed food production and the pharmaceutical industry, pervasive objection to its use with crops is irrational. There is no scientific justification for it, either from a human health or an environmental perspective.
Indeed there are examples (Golden Rice is the leading contender) where human health could benefit – both socially and economically – if genetic engineering were more rapidly adopted.
There are also environmental benefits associated with some crops created using the genetic engineering technology. Reduced use of fossil fuels in land cultivation follows the use of herbicide tolerant plants. Reduced spray applications and water usage occur as a result of insect-pest resistant plants and the higher yields thereby decrease the need to bring ever more land into cultivation to match human population growth.(114)
Acknowledgements
I acknowledge with thanks the illustrations from the sources referenced, and also other assistance from Professors P Beyer, J Gressel, and I Potrykus, and the cartoonist Duffy. I believe there are no errors, but of course if there are, they are mine alone.
