Course Authors
Peter Barland, M.D.
Dr. Barland reports no commercial conflict of interest.
This activity is made possible by an unrestricted educational grant from the Novartis Foundation for Gerontology.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the mechanisms underlying hyperuricemia and the increased body pools of uric acid found in patients with gout
State the biochemical and metabolic factors involved in the precipitation and amelioration of acute gouty arthritis
List the treatment options for acute gout and the mechanism of action of colchicine.
Introduction
The common facts of gout are well known. The goal of this conference is to review some of the recent advances in our understanding of the pathogenesis, diagnosis and treatment of gout that may be of interest to the primary care physician -- or to put it more succinctly, what's new about gout.
Gout is caused by the phagocytosis of uric acid crystals in synovial fluid which produces an intense but self-limited arthritis most commonly involving the first metatarsal phalangeal joint -- a condition known as podagra.
The diagnosis is confirmed by the visualization of intracellular needle-shaped birefringent crystals in aspirates of synovial fluid.
Figure 1.
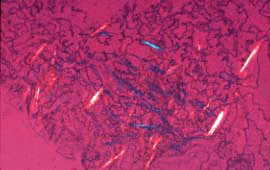
Synovial fluid with sodium urate crystals, polarized light with red compensator microscopic.
Courtesy of Gower Medical Publishing Ltd.
From Cyberounds® 10/96 Rheumatology Conference.
Gout is frequently encountered in hyperuricemic men and may be accompanied by the development of solid masses of uric acid, known as tophi, which can be seen and palpated over the extensor surfaces of joints and can cause joint erosions, and by urinary uric acid calculi.
Colchicine can shorten the duration of an acute attack of gout and can be used to prevent recurrent attacks.
Pathogenesis
Hyperuricemia
The majority of patients with gout are hyperuricemic because of a defect in the urinary clearance of uric acid. The tubular mechanisms responsible for this decreased clearance are still not well understood. Many patients, especially postmenopausal women, develop hyperuricemia as a result of prolonged diuretic therapy. Cyclosporin A, which was used primarily in the prevention of allograft rejection and is now being used in the treatment of connective tissue diseases, including rheumatoid arthritis, can cause severe hyperuricemia with frequent attacks of acute gout and the rapid development of large tophi. The drug appears to inhibit the secretion of uric acid into the proximal renal tubule.(1)
Genetics
Approximately 20% of patients with gout actually have a primary metabolic disorder leading to the overproduction of uric acid. These patients are characterized by an early onset of gout, a high incidence of tophi and urinary calculi. In some of these patients an enzymatic defect responsible for the increased synthesis of uric acid has been identified.
The enzymatic defects include a deficiency in one of the two enzymes that recycle purines into the nucleotide pool -- hypoxanthine guanine phosphoribosyl transferase and hypoxanthine adenine phosphoribosyl transferase. In the absence of these enzymes, most of the purines that are metabolized to hypoxanthine are converted, enzymatically, by xanthine oxidase to uric acid which is a metabolic end product in all primates. Another enzymatic defect that appears to be more common than the phosphoribosyl transferase deficiencies involves an alteration in the rate limiting enzyme for de novo purine synthesis -- phosphoribosylpyrophosphate glutamine transferase -- that makes the enzyme less inhibitable by nucleotides such as guanine.
These genetic mutations account for only a small fraction of the patients who overproduce uric acid. The etiology for the remaining overproducers is still unknown but may represent partial deficiencies of these same enzymes either by different mutations or as heterozygotes. In support of this hypothesis is the finding that the phosphoribosyl transferase deficiencies in different families are caused by different point mutations in the genes for these enzymes.
An Acute Attack
Crystals of uric acid can frequently be seen extracellularly in the synovial fluid of hyperuricemic patients during the intercurrent periods between attacks of gout. It is now believed that the crystals must first be coated with immunoglobulin before they will be phagocytosed (i.e., opsonized). There is some evidence that some of the immunoglobulin coating the crystals is antibody directed against or specifically reactive with uric acid.(2) The intracellular mechanisms responsible for neutrophil degranulation and release of superoxide radicals appear to involve the activation of the enzyme phospholipase D.
The chemotactic factor produced by white cells after phagocytosis of the crystals that augments and perpetuates the inflammatory response appears to be a cytokine--IL8. Almost all attacks of gout resolve while the patients are still hyperuricemic and have normal numbers of circulating polymorphonuclear leukocytes and adequate bone marrow reserves of leukocytes. The mechanisms responsible for the self-limited nature of acute gouty arthritis remain unclear. A serum factor, apolipoprotein B, appears to be capable of coating uric acid crystals and inhibiting their phagocytosis. In addition, local factors, which have not been clearly identified, are produced in the gouty joint after 48 hours and are capable of inhibiting crystal induced inflammation.
Differential Diagnosis
Crystals of calcium pyrphosphate (CPP) can cause an acute arthritis resembling gout -- a condition known as pseudogout. In contrast to gout, where the first metatarsal phalangeal joint is most commonly affected, pseudogout more frequently involves the knee or the wrist and the patients are normouricemic. The differential diagnosis is best made by the identification of the CPP crystals in polymorphonuclear neutrophils in the synovial fluid of the inflamed joint. The CPP crystals are short and rhomboid in shape and are positively birefringent in the polarizing microscope as opposed to the uric acid crystals which are negatively birefringent. The CPP often deposits in the articular cartilages and menisci, resulting in the radiological finding of chondrocalcinosis which may lead the physician to suspect CPP arthritis rather than gout.
Basic calcium phosphate (BCP), otherwise known as hydroxyapatite, may also elicit intra-articular inflammation as well as periarticular inflammation in the form of bursitis and tendinitis. This inflammation tends to be less intense and more chronic than that seen in gout and CPP arthritis but is more likely to result in erosion of the articular cartilage and underlying bone. The BCP crystals are amorphous in shape and are not birefringent and therefore cannot be seen in the polarizing microscope. The condition can be suspected by the soft tissue calcification seen on routine x-rays. A more extensive discussion of CPP and BCP arthropathies will be the subject of a future conference.
Unusual Clinical Presentations
In addition to the typical acute attack, both gout and pseudogout can be associated with pyogenic infections of the joint. Therefore, for all febrile patients or patients with atypical features, the synovial fluid should be Gram stained and cultured.
Both gout and pseudogout can occasionally present with hemarthrosis, so crystal arthritis should be on the differential of a bloody synovial fluid. Recently, erosive tophaceous arthritis of the interphalangeal of the hands has been recognized in elderly patients and is often mistaken for erosive osteoarthritis. The majority of these patients has been women on diuretic therapy and many have mild renal insufficiency. Most of these women do not have a history of podagra so that the diagnosis of gout may not be suspected.
In addition to the usual sites of tophaceous deposits (helix of the ear, over the extensor surfaces of joints and in the olecranon bursae), tophi have also been noted frequently in the finger pads of patients with tophaceous gout.
Treatment
Acute Attack
The use of oral colchicine for the treatment of an acute attack of gout has been largely abandoned. Antiinflammatory doses of a nonsteroidal antiinflammatory agent (NSAID), often with an initial extra dose on the first day of treatment, is now the treatment of choice.
Many patients, however, have contraindications to NSAID administration -- such as a history of gastric bleeding or renal insufficiency. These patients may be treated with short courses of corticosteroids or intravenous colchicine. When using colchicine it should be remembered not to exceed 4 mg in any 72-hour period.
Prophylaxis
Daily doses of colchicine, 0.6 mg bid, can significantly decrease the frequency of acute attacks and is indicated in most patients who have had more than one attack of gout per year. New evidence suggests that colchicine acts, at these low doses, by altering the display of adhesion molecules (E-selectin) on the surface of activated endothelial cells.(3) These molecules normally react with ligands on the surface of circulating neutrophils and, thereby, control egress of inflammatory cells in response to chemotactic stimuli. Allopurinol, an inhibitor of xanthine oxidase, can effectively reduce the synthesis of uric acid. The use of allopurinol should be limited to patients who are overproducers, have a history of urinary calculi, tophi or recurrent gouty attacks despite colchicine prophylaxis and have persistent hyperuricemia with levels of > 9mg/dL. A schedule for desensitizing patients with allopurinol allergy has been published.(4)
Summary
Gout is one of oldest and best understood of all the human metabolic disorders but new observations continue to evolve. These observations include: the presence of serum factors such as apolipoprotein B which inhibits the initial phase of gout -- the phagocytosis of uric acid crystals; serum factors such as immunoglobulins specific for uric acid which promote phagocytosis; synovial factors such as IL 8 which amplify the immune response and other synovial factors which dampen the inflamatory response. All of these factors represent potential targets for treatment and prophylaxis of gout. Cyclosporin and diuretic induced tophaceous gout represent newly recognized variants of gout and the effect of colchicine on endothelial cell adhesion molecules may open up new pharmacological approaches to the prophylaxis and treatment of this disorder.