Course Authors
Calvin H. Hirsch, M.D.
Dr. Hirsch is Professor of Clinical Internal Medicine and Public Health Sciences, Division of General Medicine, UC Davis Medical Center, Sacramento, CA.
Within the past 12 months, Dr. Hirsch reports no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff, and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss how exercise fits into the care of older patients as primary, secondary and tertiary prevention
List the strength and limitations of current evidence supporting the role of exercise for specific medical conditions
Screen patients' levels of physical activity and physical functioning using validated instruments
Counsel patients regarding exercise based on the patient's readiness to become active.
"Exercise: the Universal Antidote for Aging" appeared as the title of an October 2009 article in the Johns Hopkins Medical Letter: Health After 50.(1) One might dismiss the title as reminiscent of tabloid hype for "cure-all" nostrums were it not for solid evidence which shows that exercise reduces the risk of cardiovascular and all-cause mortality; reduces risk for breast, colon and possibly prostate cancer; improves physical function and slows functional decline in general and in specific diseases like knee osteoarthritis; prevents and treats type 2 diabetes mellitus; reduces fall risk; and lowers the risk of Alzheimer's disease.(2)
The importance of exercise for seniors is reflected in the 2008 Physical Activity Guidelines for Americans,(3) which recommend that older adults engage in at least 75 minutes of vigorous, aerobic physical activity or 150 minutes of moderate-intensity activity per week, together with muscle-strengthening exercises two or more days per week. However, 69.8% of U.S. adults ≥age 65 are either sedentary or insufficiently active to derive health benefits from their exercise.(4) Between the National Health and Nutrition Examination Surveys of 1988-1994 and 1999-2004, a 35%, 40% and 42% decline in reported monthly physical activity occurred in the age groups 60-69, 70-79 and 80+, respectively, along with increases in disability in activities of daily living (ADL) and motor function in persons age 60-79.(5)
Physical inactivity among older persons is both a public health concern and an important clinical condition that can affect an individual’s functional status, quality of life and the management of chronic disease. While inactive seniors are potential candidates for counseling and interventions aimed at increasing their level of physical activity, how and for whom this is done involves multiple, often complex issues that can determine the feasibility and effectiveness of the effort (Figure 1).

Figure 1. Clinical decision-making involved in the development of an exercise prescription.
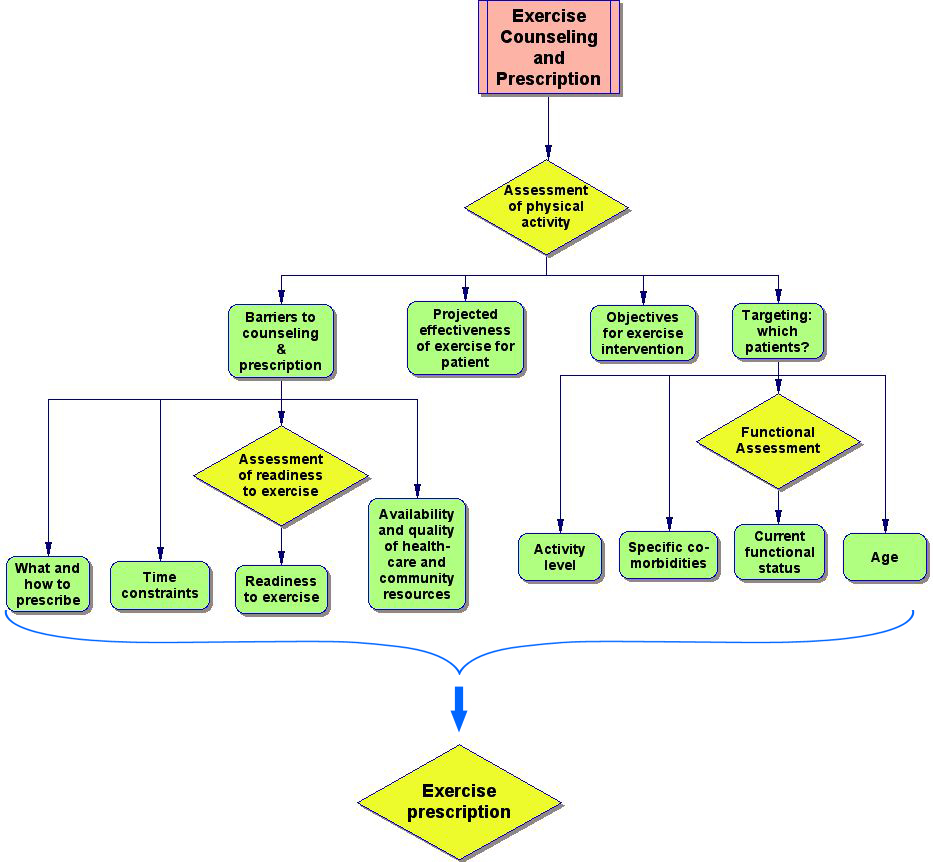
Click image to view larger.

Exercise fits into clinical care as primary, secondary and tertiary prevention (Table 1). For the individual at high risk for developing cardiovascular disease, diabetes, obesity and certain cancers, exercise should be integrated into a program of multiple risk-factor reduction, but the amount of risk reduction conferred by exercise alone cannot be known. Patients with disease or impairment, such as knee osteoarthritis or difficulty arising from a low chair, are more likely to perceive the benefits of exercise that targets these conditions. Whether exercise is "marketed" to sedentary seniors as prevention or as treatment may affect their readiness for, and adherence to, the exercise program.

Table 1. Type of Prevention.
| Primary | Secondary | Tertiary | |
|---|---|---|---|
| (Risk Reduction) | (Treatment) | ||
| Illness | Absent | Absent | Present |
| Disease or Age-Associated Decline or Impairment | Absent | Present | Present |
Examples
|
Examples
|
Examples
|
|

This Cyberounds® will review the diverse effects of exercise on the health of older persons, as well as evidence-based approaches to the assessment of physical activity and physical performance. Finally, this Cyberounds® will discuss challenges in prescribing exercise for patients. We use the terms "physical activity" (kilocalories expended in muscle work) and "exercise" (kilocalories expended for the purpose of improving or maintaining physical performance) interchangeably.
Primary Prevention: Reduction in Mortality and Incident Disability
Higher physical activity has been significantly associated with a lower risk of cardiovascular and all-cause mortality,(6)(7)(8)(9)(10)(11)(12) but in older persons supporting data have been derived exclusively from observational cohort studies. Despite controlling for covariates, these studies are limited by an unmeasured-health bias — those who were active may have been so because they were healthier than sedentary individuals in ways that were not assessed. However, the patterns of data collection in some cohort studies may permit a low-bias analysis that mimics the design of a randomized, controlled trial.(13) Applying this analytical approach to healthy, sedentary participants of the Cardiovascular Health Study (CHS), a cohort study of 5,288 persons age 65 and older, those who became active after two years of observation (defined as increasing their walking to at least 28 blocks per week) had a significantly lower mortality and fewer hospitalizations over the succeeding two years than participants who remained sedentary, after adjustment for age and health characteristics.(13)
The CHS findings are consistent with earlier data from the Study of Osteoporotic Fractures (SOF) that showed that older women who took up exercise had similar reductions in the hazard-rate ratios for all-cause and cardiovascular mortality, over a mean follow-up of 6.7 years, as women who were always active. While the data support the survival benefits of the late adoption of exercise, the SOF trial also found that the survival benefit of exercise for women who stop being active rapidly disappears over several years, and apparently does not depend on the long-term, cumulative effects of exercise on health.(7) These findings underscore the importance of encouraging active seniors to continue exercising.
The amount of risk reduction for death or ADL disability that can be attributed to exercise varies inversely with age. In the CHS, the survival advantage (over 11 years of follow-up) associated with physical activity in persons over age 64 accrued primarily to men of advanced old age (75+) who expended at least 1,800 kcal/week in recreational and household physical activity. The lack of association between physical activity and survival in the young-old and in women may have reflected, in part, their low overall mortality rate and, in women, lower levels of physical activity than among men. The survival advantage was also small: among men ≥age 75, the most active participants lived just 1.5 years longer than the least active group (95% CI 0.79, 2.19 years) after extensive adjustment for demographic and health-related covariates. By contrast, the greatest relative gain in years of life free of ADL disability (over six years of follow-up) was found in the most active quintile of women aged 75 and older compared to their sedentary counterparts (1.4 years [95% CI 0.94, 1.78]). The most active men in this age group averaged only 0.57 more years of ADL independence (95% CI 0.19, 0.95) than sedentary men.(8)
Secondary and Tertiary Prevention: Strength Training to Prevent Age-associated Functional Impairment
Aging is associated with a progressive decline in muscle mass (sarcopenia) and a disproportionate loss of fast-twitch (Type II) muscle fibers, contributing to a progressive decline in motor performance. Between 12% and 15% of men and women aged 60-65 demonstrate very low grip strength and gait speed, and the proportion exceeds 40% by the ninth decade.(14) These age-associated changes have practical implications. At 141 cross-walks sampled in the U.S., the minimum speed required to cross the street before the light changed averaged 0.49 (± 0.20) meters/second.(15) A gait speed of 0.7 m/s, only slightly slower than the average octogenarian’s, represents a practical threshold of clinical concern for older patients. Because they achieve lower peak strength than men, aging women are more likely to cross the threshold into impairment (difficulty performing muscle-related activities) and ultimately disability (inability to perform these activities) earlier than men.
Lower-extremity performance measures that require integrated neuromuscular coordination, such as gait speed, stride length, ability to climb stairs and balance — can be influenced by multiple, often interacting, factors in addition to motor weakness, including: musculoskeletal pain; cardiovascular, pulmonary and neurological co-morbidities; age-associated sensory changes; and the presence of subclinical white matter disease.(16)(17)(18) Nevertheless, poor lower-extremity strength remains a powerful, independent predictor of future mobility disability.
Among participants in the Italian Invecchiare in Chianti (inCHIANTI) Study of Aging, men with knee extension strength below 19.2 kg and women with knee extension strength below 18.0 kg experienced a roughly four-fold greater three-year decline in gait speed than men and women above the respective strength thresholds. Knee extension power <105 watts (W) in men and <64 W in women was associated with an approximately 50% incidence of mobility disability over three years (defined as loss of the ability to walk 1 km or climb stairs), compared to 5.7% and 15.7% in men and women, respectively, whose leg power exceeded these thresholds. Persons over age 74 experienced the greatest loss of gait speed and the highest incidence of mobility disability.(19)
Intensive resistance training, even in the very old (≥85), leads to selective hypertrophy of fast-twitch type II fibers, with a relative increase in the percentage of type IIa versus type I fibers.(20) Such a change theoretically should improve muscle power, not just strength, and it appears that increases in power, rather than strength alone, may be responsible for improvements in gait speed and composite measures of physical performance that can be seen in exercise interventions.(2) Among 53 completers in the exercise arm of a nine-month, randomized, controlled trial of exercise for community-dwelling, sedentary, frail seniors, total body fat-free mass and leg lean mass each increased just under 2%, but knee-extension strength increased by 8%.(21) This study demonstrates that small increases in lean mass translate into proportionately greater improvements in muscle performance.
Although a high level of physical activity, versus being sedentary, has been associated with up to a 70% reduction in the relative risk of incident ADL disability and up to an 82% reduction in the relative risk of incident Instrumental Activities of Daily Living (IADL) disability over three years,(22) data from observational cohort studies may overestimate the benefits of exercise due to the healthy-subject bias described above.(13)
While we await the results of the FRAilty, Screening and Intervention (FRASI) randomized trial of exercise to prevent ADL disability in frail seniors,(23) frailty based on a score ≥9 on the Short Physical Performance Battery (SPPB)(24) published clinical trials only provide data on the effects of exercise on physical performance. Liu and Latham performed a series of meta-analyses on 121 clinical trials of progressive resistance training (PRT) involving a total of 6,700 older subjects.(25) The majority of trials used moderate-intensity training with a frequency of two to three times per week and a duration between 8 and 16 weeks. Overall, strength training produced significant gains in muscle strength that translated into statistically significant but small gains in gait speed that have questionable clinical significance (weighted mean difference between intervention and control groups = 0.08 m/s (95% CI 0.04 to 0.12), as well as minor gains in endurance, as measured by the six-minute walk test. PRT also had modest beneficial effects on the clinically relevant speeds of chair-stands and stair-climbing.
While the meta-analysis of Liu and Latham offers enhanced statistical power by combining subjects from multiple trials, the results still have to be viewed cautiously for clinical relevance because of the inherent heterogeneity of subjects and the specific interventions used. Well-designed smaller trials can provide insight into whether exercise interventions, which are within the scope of community-based practice, can provide clinically meaningful benefits to frail seniors. Short-term home physical therapy (PT) for frail community-dwelling older persons that focused on improving indoor mobility and gait; reducing the presence of environmental hazards; and providing a progressive exercise program designed to be continued without supervision resulted in a significantly smaller increase in ADL disability scores after 12 months when compared to a control (educational intervention).(26) Moderately frail defined as requiring >10 s to walk a 3-meter course, turn around, and come back or inability to stand up from a hard chair without pushing off with their hands. Inability to do both qualified the subject as severely frail.(26) This modest intervention also improved practical performance skills. Compared to the control group at 12 months, the PT group experienced half as much loss in rapid gait speed, over twice as much improvement in the ability to arise from a chair and over three times the ability to quickly perform essential tasks like taking a jacket on and off and picking up an object from the floor.(27)
The PT intervention slowed disability progression only among those participants who were moderately – but not severely – frail, suggesting that non-intense exercise interventions may be insufficient – or too late – to stave off the progression of functional impairment that is already advanced.(26) This and earlier research by Gill et al.(28) utilized a linear scale to score ADL disability. The scale assigned a point for difficulty with a component ADL like bathing and two points for requiring help with that activity, regardless of difficulty. An increase of just two points on this 16-point scale (0=fully independent, 16=completely dependent in all ADL) predicted nursing home admission or death within three years.(28) More discussion of clinically useful ways to screen for functional impairment can be found below.
Exercise to Prevent and Treat Specific Conditions
Falls: Over one-third of persons over age 65 fall each year, with potentially catastrophic consequences, including hip fracture, permanent loss of independence, institutionalization and death.(29) Fear of falling can result in self-imposed restrictions on physical activity,(30) leading to a downward spiral of worsening lower-extremity strength, gait instability and further restrictions of activity that worsen fall risk. The risk for falling commonly has multiple contributors, and these risks can be categorized as "intrinsic" (e.g., muscle weakness, visual impairment, cognitive impairment) and "extrinsic" (e.g., polypharmacy, environmental hazards like throw rugs and lamp cords). The concurrence of just three risk factors – hip weakness, balance instability and taking ≥4 medications – has been associated with a 100% occurrence of falls after one year in a cohort of older subjects.(31)
Muscle weakness (whether measured by lower-extremity strength testing or by grip strength) was the most important risk factor for falls in a review of sixteen studies (RR/OR = 4.4 [95% CI 1.5, 10.3]), ahead of a history of falls, impaired gait, poor balance and the use of an assistive device.(32) As a consequence, the American Geriatrics Society (AGS), the British Geriatrics Society (BGS and the American Academy of Orthopaedic Surgery (AAOS) Panel on Falls Prevention recommended exercise programs with balance training as an integral part of a multifactorial intervention for community-dwelling older persons at high risk of falling.(32) The pooled results of a recent meta-analysis of 18 studies that utilized an exercise or physical therapy intervention to prevent falls showed a modest 13% reduction in fall risk compared to control groups (RR = 0.87, 95% CI 0.81-0.94).(33) Seventeen of the 18 studies also included some form of gait, balance or functional training.
Translating these results into clinical practice is challenging. Despite the strong evidence supporting exercise as means to prevent falls, there remains uncertainty over what forms of strength training work best (progressive resistance training for the legs, walking, stationary cycling, etc.), how intense the exercises should be, how long the exercise program should last and the best methods to improve balance.
A version of Tai Chi C’uan, modified for seniors, has been shown to reduce fall risk.(34) Compared to a control group that did only stretching exercises, older women receiving either agility training or resistance training achieved similar, statistically significant reductions in a fall risk score, although the incidence of falls was similar in all three groups.(35) Because this study was not powered to compare fall incidence, it is premature to recommend agility training or strength training alone for fall prevention.
The AGS, BGS and AAOS have recommended a long-term exercise and balance-training program for recurrent fallers, although this recommendation is based on expert opinion rather than hard data. It is also unclear how much of the exercises (including gait and balance training) should be conducted in a supervised environment by trained instructors and when or if older patients can safely perform these exercises on their own. The effectiveness of these interventions for actual patients will vary because of the heterogeneity of exercise interventions and the variability of community-based therapists and trainers. Consequently, it is incumbent on clinicians to develop and maintain their own "social PDR" (Physicians’ Desk Reference) of reputable community exercise programs, as well as to identify physical therapists with interest and expertise in fall prevention.
Diabetes mellitus: The ratio of lean (muscle) mass to fat mass declines with normal aging, contributing to insulin resistance. In the United States, these age-associated changes, combined with the rising prevalence of overweight or obese adults, have resulted in an increase in the prevalence of diabetes among persons ≥65 from 20% to 27% in the past decade.(36) Taking up exercise in middle age can prevent the development of diabetes. Among 3,234 non-diabetic persons (mean age=50) with elevated fasting and post-glucose-loading blood sugars, those randomly assigned to intensive lifestyle counseling both increased their level of physical activity during the four years of follow-up and had a significantly lower incidence of diabetes, compared to controls (4.8 cases/100 person-years v. 11.0 cases per 100 person-years).(37)
Both aerobic exercise and strength training can improve glycemic control in patients who already have diabetes, although clinical trials have focused mainly on middle-aged adults.(38)(39) In a meta-analysis of nine clinical trials assessing the effects of exercise interventions on glycosylated hemoglobin (HbA1C), the exercise groups averaged a 0.66 drop in their HbA1C (95% CI 0.34, 0.98) compared to sedentary subjects.(38) The sustainability of improvement in HbA1C after an exercise intervention, and its long-term effect on diabetic complications, remain unknown, but likely depend on a permanent lifestyle change favoring exercise.
Clinically meaningful, long-term improvement in physical activity can be achieved (at least in middle-aged adults) when lifestyle counseling is personalized, culturally sensitive, tied to achievable goals and continued for at least six months.(37) In 40 women and 22 men with a mean age of 66 (±8), a sixteen-week program of moderately intensive resistance training resulted in a significant one-percentage-point drop in HbA1C and a significant increase in muscle glycogen stores, compared to control subjects. Seventy-two percent of the exercise group was able to reduce the dose of prescribed hypoglycemic medication.(40) In one clinical trial, supervised brisk walking was associated with a significant reduction in HbA1C and fasting glucose only among subjects who attended at least half of the exercise sessions, emphasizing the point that exercise, like any prescribed medication, will not work without adherence.(41) Older diabetics are less likely to follow current exercise guidelines than their non-diabetic peers, and female gender, age ≥75, being African-American and obesity all independently predict non-adherence.(42)
Osteoarthritis of the knee: The incidence of knee osteoarthritis (OA) rises exponentially after age 50 to a peak of approximately 1100 per 100,000 person-years in women and approximately 850 per 100,000 person-years in men during the 8th decade.(43) As knee OA progresses, it can cause pain, impair mobility and increase difficulty in performing daily activities, culminating in disability and reduced quality of life.
The Fitness Arthritis and Seniors Trial (FAST)(44) randomized 439 community-dwelling seniors ≥age 60 (mean age=69) with symptomatic knee OA to one of three groups: aerobic exercise (walking), resistance training and health education. Each exercise intervention involved three months of supervised group activity, followed by 15 months of home-based exercise with periodic home visits and telephone contacts from the exercise leader. Those assigned to resistance training performed exercises that strengthened major muscle groups of both the upper and lower extremities. At the end of 18 months, the two exercise groups had similar disability scores that were significantly lower than the education group’s (Figure 2).

Figure 2. Adjusted means with standard errors for 3 groups in the FAST trial of exercise for knee OA.
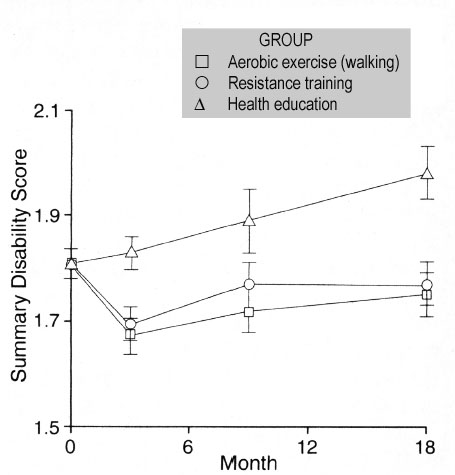
Disability scores range from 1 (no difficulty) to 5 (unable to do). At month 18, the aerobic exercise group and resistance-training group had significantly lower scores than the education group (p<0.001 and p=0.003, respectively). Adapted from Ettinger et al., 1997.(44)

Compared to the education group, the two exercise groups also reported less knee pain and had better scores on performance tasks like getting out of a car and the distance walked in six minutes. Not surprisingly, adherence to the exercise program correlated with the scores for disability and pain intensity, with the lowest adherence tertile reporting the most disability and pain, as well as showing the shortest distance walked in six minutes. Among the 250 FAST participants free of ADL disability at baseline, the cumulative incidence of ADL disability after 18 months was 15.4% lower in the exercise groups than in the educational group (37.1% v 52.5%, p=0.02). The two exercise interventions conferred similar risk reductions for ADL disability.(45) Other clinical trials have shown similar results that confirm the efficacy of exercise interventions to reduce pain and disability scores in patients with knee OA.(46)
The evidence overwhelmingly supports the addition of exercise – aerobic and/or strength-training – to the treatment plan of knee osteoarthritis, and exercise for knee OA has received strong endorsement from the Osteoarthritis Service and Integration System (OASIS) group based on the high quality of the data.(47) These studies also dispel the myth that walking as exercise is contraindicated because it can exacerbates knee OA. The OASIS group also concluded that the choice of exercise should be based on availability, preference and tolerance, although certain sports may increase the risk of knee OA, depending upon the intensity and duration of participation in that sport and the risk of sustaining knee trauma (e.g., skiing and soccer).
Cognitive decline: Population-based studies have shown that higher levels of physical activity reduce the risk of Alzheimer's disease, vascular dementia and all-cause dementia,(48)(49)(50)(51)(52)(53) although the relationship between physical activity and dementia has not been found consistently.(54) More recently, an interaction between the level of physical functioning and physical activity has been observed, such that those with worse physical function who nevertheless engage in higher levels of physical activity experience the largest reduction in the risk of dementia.(49)(52) Although the etiology of this association remains speculative, cerebral white matter disease affects stride length and gait speed,(18)(55)(56) and white matter changes have been associated with cognitive performance.(57) In cross-sectional studies of non-demented older persons, higher physical activity has been associated with better executive function and a lower prevalence of mild cognitive impairment.(58)(59) The protective effect of physical activity appears to be limited to those who do not carry the APOE ε4 allele.(51)
There are few data regarding the cognitive benefits of taking up exercise in old age. A year-long, moderate-intensity aerobic training program among 87 sedentary older persons found that the exercise group maintained their performance on the Wechsler Memory Scale, while the control group’s scores decreased.(60) Eighteen months after 170 non-demented older persons with complaints of memory loss (mean age=69) were randomized to a 24-week home-based physical-activity program or to a control group, those in the exercise arm experienced a significantly smaller decline on the Alzheimer's disease Assessment Scale — Cognitive Subscale (ADAS-Cog) than the control group (-0.73 [95% CI, -1.27, 0.03] v. -0.04 [95% CI -0.46, 0.88], p=0.04).(61) These data suggest that adoption of a physically active lifestyle may have some benefit in slowing decline in certain domains of cognitive function, but it remains unknown whether it can prevent or forestall the development of dementia. A clinical trial is under way to determine if exercise can reduce behavioral problems in dementia.(62)
How Much Exercise Is Enough?
Data from observational cohort studies generally demonstrate a dose-response effect for the benefit of exercise on preventing outcomes like cardiovascular and all-cause mortality, as well as functional dependence. Cohort studies thus suggest that more is better, but the more strenuous the program, the more likely that patients will either refuse to participate or drop out. Fortunately, the absolute amount of exercise needed to produce a significant reduction in risk (compared to being sedentary) declines with increasing age.(8)
Most clinical trials of exercise in older adults have employed moderate-intensity regimens that are feasible for community-dwelling seniors. Walking is the most common and acceptable form of leisure-time physical activity,(63)(64) and as little as 28 blocks per week (roughly equivalent to 1.4 miles) have been shown to reduce the two-year relative risk of mortality, dementia, depression and ADL dependence compared to remaining sedentary.(13) However, it appears that intensity, or briskness, of walking plays a more important role in its beneficial effects than the overall time spent.(65) In multivariate analyses using data from the 73,743 post-menopausal participants age 50-79 from the Women’s Health Initiative, self-reported walking and vigorous exercise (defined as working up a sweat and causing the heart to beat fast) were associated with similar reductions in the risk of cardiovascular events. The authors also found that fewer hours spent sitting each day, as well as the briskness of walking, independently lowered cardiovascular risk.(9)
Screening for Exercise and the Exercise Prescription
General activity screening: In Europe, the public health hazards of a sedentary lifestyle are well-recognized. Acknowledging the importance of a physically active lifestyle for the United Kingdom, the Department of Health (UK) has embarked on an ambitious program called Let’s Get Moving,(66) whose goal is to engage general practitioners and local primary-care trusts, organizations in the United Kingdom whose purpose is to develop health services for a particular community, to increase patients’ physical activity through the expansion and integration of services facilitating physical activity such as local County Sport and Physical Activity Partnerships and the publication of local walking maps. The primary care physician plays a central role by screening patients’ level of physical activity, providing exercise counseling, disseminating exercise guidelines and, when appropriate, referring to a local exercise program or an exercise specialist (so-called "exercise on prescription"). Let’s Get Moving also provides training in exercise counseling to general practitioners.
Even with an infrastructure to support Britain’s exercise on prescription (EOP) program, long-term adherence to prescribed physical activity is modest. In one study of 449 sedentary individuals (mean age=57) receiving an exercise prescription, 27% had dropped out by 4 months, 52% by 10 months and 66% by 16 months.(67) Although an exercise prescription is not a panacea for the current epidemic of physical inactivity, it is a useful tool, especially since most older individuals come into contact with a primary care provider, who, therefore, is in a unique position to assess and encourage physical activity.(68)(69)
Physician activity assessment, counseling and "prescription" (or referral) can be done efficiently without disrupting normal patient flow. In the Activity Counseling Trial (ACT), 54 physicians or physician assistants from 11 primary care practices in three states were trained to integrate three to four minutes of physical activity assessment and counseling into the routine office visits of sedentary patients aged 35-75. When surveyed, 63% of the practitioners reported that the extra activity counseling minimally added to visit times; 83% of the healthcare personnel devoted less than six minutes to counseling; and 46% provided the recommended three to four minutes.(70)
The actual ACT intervention consisted of three arms that differed in the intensity of counseling provided by a health educator upon referral by a study physician. At 24 weeks, no significant differences in outcomes were observed among the three intervention groups, which were combined for analysis. Noteworthy was a consistent, strong interaction between exercise counseling and the baseline levels of cardiac risk factors & only subjects with abnormal baseline values of the risk factors showed improvements in the risk factors. In multivariate analyses, change in weight, but not fitness, was associated with risk-factor improvement.(71) The ACT trial demonstrates that a modest investment of clinician time in activity counseling is both feasible in outpatient practice and potentially effective for improving intermediate outcomes like cardiac risk factors.
For efficiency, counseling should preferentially target patients who are sedentary or are at risk for adverse outcomes like falls. Little attention has been paid in the literature to the validity and reliability of activity screening questionnaires for practicing clinicians, who need short, easy-to-use instruments that are self-explanatory and can be completed by the patient prior to the encounter. The one-page instrument used in the ACT trial,(70) the General Practice Physical Activity Questionnaire from the UK National Health Service(72) and the Family Practice Activity Screen (FPAS)(73) represent feasible options that can be readily adopted into clinical practice. Each questionnaire is provided in Appendix 1.
The ACT questionnaire separates activities into light, moderate and hard and seeks to quantify the amount of time spent in each category of activity. It does not directly define what constitutes being sedentary, although inadequate physical activity easily can be inferred. The instrument’s psychometrics are not provided, and ACT is not specifically geared toward older patients. ACT is, however, very useful because it indirectly assesses motivation to exercise by asking the respondent to check off up to nine reasons to be active.
The UK Department of Health developed the General Practice Physical Activity Questionnaire (GPPAQ) as part of its national Let’s Get Moving program. The one-page GPPAQ consists of three questions. The first asks about the respondent’s type of work; the second inquires about the amount of time per week spent in specific activities; and the third asks about gait speed. Although its psychometric properties are not provided, the questionnaire has been carefully developed for the country’s National Health Service and targets adults of all age groups. An algorithm allows the questionnaire to be scored "active" or "inactive," and for practitioners an Excel™ spreadsheet version performs this calculation automatically. Both the pdf and spreadsheet versions are available online(72). The spreadsheet permits tabulation of activity data on multiple patients, making it suitable for continuous quality improvement (CQI) projects.
The FPAS is available as two-question and three-question versions, which have moderate test-retest reliability (kappa 58.0 and 55.6, respectively) but poor-fair criterion validity based on accelerometer data (kappa 18.2 and 24.3).(73) Both are designed to be administered by the clinician, but can be completed in just one to two minutes. Published experience with this activity screen is lacking.
Assessing readiness to become active: Activity counseling should be adjusted for the patient’s readiness and motivation to exercise. The Transtheoretical Model provides a conceptual framework to understand where the patient fits on the continuum of preparedness to change behavior. The model starts with pre-contemplation (not even thinking about it), moves to contemplation, then to preparation for change, next to actively changing behavior, and finally to maintenance of that new behavior.(74) Applying this model, Marcus et al. identified two principal components from a 40-item exercise questionnaire. One component, consisting of six items, represents reasons not to exercise and the second component, comprising 10 items, represents favorable perceptions of exercise. A "decisional balance measure" (pros minus cons) correlated significantly with the adoption of the stage of change.(75) Incorporation of this simple questionnaire (Appendix 2) into pre-visit screening may offer a more accurate estimate of a patient’s willingness to exercise and may help direct activity counseling. When counseling, the word "exercise" should be used cautiously, as it can have unpleasant connotations for some individuals, who may equate "exercise" with structured physical activity like gym class that requires specialized clothing and footwear, and entails getting hot and sweaty.
Assessment of functional status and fall risk: The need for exercise counseling and prescriptions extends to individuals who are experiencing difficulties with daily activities. Instrumental and basic activities of daily living such as the Lawton IADL76 and Katz ADL(77) provide useful information, particularly if the degree and causes of difficulty are assessed. (See Appendix 3.) Questions borrowed from aging cohort studies can be used to screen for mobility and upper-extremity impairment: How much difficulty do you have…
- Walking ½ mile (1 km)?
- Climbing 10 steps?
- Lifting 4.5 kg (10 lbs)
- Reaching for objects?
For determining fall risk, a comprehensive evaluation requires inquiry into recent falls and their circumstances, assessment of visual acuity, review of medications and medical conditions that could contribute, and a careful neuromuscular examination. Validated fall-risk screening tools can improve the sensitivity of fall-risk detection. The simplest tool is the Up and Go Test, which involves having the patient stand up from a chair (if possible, without using the hands to push off), walk three meters, turn, walk back and sit down. If the patient regularly relies on an assistive device for walking, it should be used during the test. Observing the ease of standing, initial balance and stability of the gait provide useful qualitative information. Timing the Up and Go Test – with the patient walking as quickly as possible – adds an easy but powerful predictive tool. A cut-off score of ≥13.5 seconds to complete the task has a sensitivity of 80% and a specificity of 100% for predicting falls.(78) An accurate qualitative assessment of gait and balance requires some training and experience. The well-validated Tinetti Gait and Balance Scale (Appendix 4)(79) breaks down balance and gait into individual components which are scored and summed to produce a quantitative fall risk. Systematic evaluation with the Tinetti Scale will, over time, allow clinicians using the Up and Go Test to more accurately assess fall risk.
Exercise Risks
Good clinical judgment should be used to assess risk for harm caused by any exercise program recommended to the patient. Most clinical trials of exercise for older persons have published little information on the incidence of morbidity caused by the exercise. A meta-analysis of nine trials of fall prevention did not find any reported increase in falls or other major adverse events.(33) In the Fitness and Arthritis Seniors Trial (FAST), only 2% of participants experienced serious exercise-related musculoskeletal injuries.(44) The brief Exercise and Screening for You (EASY) questionnaire(80)(81) has been developed for older persons considering exercise. EASY help seniors recognize health problems that could increase the risk of harm from increased physical activity and encourages those at risk to consult a physician prior to beginning an exercise regimen. For patients with symptomatic or significant coronary heart disease, heart failure or pulmonary disease, highly structured cardiac rehabilitation programs can provide closely monitored, custom-tailored, progressive aerobic training.(82) Enrollment requires a formal referral from a physician; some programs accept debilitated, older patients who require closely monitored exercise but do not have active cardiopulmonary disease.
Summary
Avoidance of a sedentary lifestyle and targeted exercise interventions for seniors have the potential to improve physical performance, prevent or delay disability, prevent falls, improve glycemic control, slow cognitive decline, reduce disability and pain from knee osteoarthritis, and even prolong life. Clinicians can and should play a key role in assessing patients’ levels of physical activity and in counseling them regarding the benefits of physical activity. Exercise prescriptions, generally involving referral to a community exercise program or to a physical therapist, constitute an important pathway to enhancing the patient’s physical functioning and quality of life.

Appendix 1.

Appendix 2. Readiness for Exercise.
| Agree | Disagree | |
|---|---|---|
| Pros | ||
| I would have more energy for my family and friends if I exercised readily. | ||
| Regular exercise would help me relieve tension. | ||
| I would feel more confident if I exercised regularly. | ||
| I would sleep more soundly if I exercised regularly. | ||
| I would feel good about myself if I kept my commitment to exercise regularly. | ||
| Regular exercise would help me have a more positive outlook on life. | ||
| Cons | ||
| I think I would be too tired to do my daily work after exercising. | ||
| I would find it difficult to find an exercise activity I enjoy that is not affected by bad weather. | ||
| I feel uncomfortable when I exercise because I get out of breath and my heart beats very fast. | ||
| Regular exercise would take too much of my time. | ||
| I would have less time for my family and friends if I exercised regularly. | ||
| At the end of the day, I am too exhausted to exercise. | ||
Adapted from: Marcus BH, Rakowski W, Rossi JS, Assessing motivational readiness and decision making for exercise. Health Psychology 1992; 11(4):257-61.

Appendix 3. Instrumental Activities of Daily Living (self-report version).
(Adapted from Lawton MP, Brody EM. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9:179-185.)
For each question, circle the number corresponding to how much help you need (or would need).
| Activity | Score | Reason(s) |
|---|---|---|
| 1. Can you use the telephone | ||
| without help | 3 | |
| with some help, OR | 2 | |
| are you completely unable to use the telephone? | 1 | |
| 2. Can you get to places out of walking distance | ||
| without help | 3 | |
| with some help, OR | 2 | |
| are you completely unable to travel unless special arrangements are made? | 1 | |
| 3. Can you go shopping for groceries | ||
| without help (or don’t do this but could without help) | 3 | |
| with some help (or would need some help), OR | 2 | |
| are you completely unable to do any shopping? | 1 | |
| 4. Can you prepare your own meals | ||
| without help (or don’t do this but could without help) | 3 | |
| with some help (or would need some help), OR | 2 | |
| are you completely unable to prepare any meals? | 1 | |
| 5. Can you do your own housework | ||
| without help (or don’t do this but could without help) | 3 | |
| with some help (or would need some help), OR | 2 | |
| are you completely unable to do any housework? | 1 | |
| 6. Can you do your own handyman work | ||
| without help (or don’t do this but could without help) | 3 | |
| with some help (or would need some help), OR | 2 | |
| are you completely unable to use the telephone? | 1 | |
| 7. Can you do your own laundry | ||
| without help (or don’t do this but could without help) | 3 | |
| with some help (or would need some help), OR | 2 | |
| are you completely unable to do any laundry at all? | 1 | |
| 8a. Do you take medicines or use any medications? | ||
| Yes | ♦ | (If yes, answer Question 8b) |
| No | ♦ | (If no, answer Question 8c) |
| 8b. Do you take your own medicine | ||
| without help (in the right doses at the right time) | 3 | |
| with some help (take medicine if someone prepares it for you and/or reminds you to take it), OR | 2 | |
| (are you/would you be) completely unable to take your own medicine? | 1 | |
| 8c. If you had to take medicine, can you do it | ||
| without help (in the right doses at the right time) | 3 | |
| with some help (take medicine if someone prepares it for you and/or reminds you to take it), OR | 2 | |
| (are you/would you be) completely unable to take your own medicine? | 1 | |
| 9. Can you manage your own money | ||
| without help (or don’t do this but could without help) | 3 | |
| with some help (or would need some help), OR | 2 | |
| are you completely unable to handle money? | 1 | |
Basic Activities for Daily Living (BADL) Sheet For Clinical Use
Adapted from Katz Index of Activities of Daily Living. From Katz S, Ford A, Moskowitz R, et al., 1963; JAMA 185: 914-19.
Check each column that characterizes the patient. If the patient can’t perform or is having difficulty with a self-care task, ask the patient why he or she is having difficulty and who helps with the task. Patients who can’t perform or have lots of difficulty with a task, and who do not have someone who regularly assists them with it, have an unmet care need and may need referral to the social worker.
| Function | Independ -ent, no difficulty | Independ -ent but having some difficulty | Independ -ent, having lots of difficulty | Can't do | Getting some help | Getting lots of help | Who helps? | Cause(s) for difficulty or depend -ence |
|---|---|---|---|---|---|---|---|---|
| Bathing | ||||||||
| Dressing | ||||||||
| Toileting | ||||||||
| Transfer | ||||||||
| Feeding |

Appendix 4. Tinetti Balance and Gait Evaluation.*
| BALANCE | GAIT | ||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Instructions: Seat the subject in a hard, armless chair. Test the following maneuvers. Circle one number that best describes the subject's performance in each test, and add up the scores at the end. | Instructions: The subject stands with the examiner, and then walks down the hallway or across the room, first at the usual pace and then back at a rapid but safe pace, using whatever walking aid the patient is accustomed to use. | ||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||
|
|
|
Overall score interpretation:
< 19: High fall risk;
19-24: moderate fall risk;
> 24: Low fall risk
*Adapted from: Tinetti M. Performance-oriented assessment of mobility problems in elderly patients. J Am Geriatr Soc 1986; 34: 119-126
