Course Authors
Barry A. Weissman, O.D., Ph.D.
Barry A Weissman, O.D., Ph.D., is Professor of Ophthalmology and Chief, Contact Lens Service, Jules Stein Eye Institute, and Department of Ophthalmology, David Geffen School of Medicine at UCLA, and Adjunct Professor of Optometry, Southern California College of Optometry, Fullerton CA.
Within the past 12 months, Dr. Weissman reports receiving grant/research support from The Vision Care Institute, and has been on the Speakers Bureau for Abbott Medical Optics, Alcon, Bausch and Lomb, Vision Service Plan and Vistakon.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Classify and diagnose the various potential complications of contact lens wear
Understand that the most serious complications of contact lens wear are both microbial corneal infection and corneal neovascularization as both can threaten vision
Apply a treatment plan for each of the CL complications
Recognize that hypoxia, once the "king" of CL-driven complications now has only a secondary role to inflammatory complications with the popularization of new highly oxygen permeable rigid and soft lenses.
Disclaimer
All effort has been taken to be sure that diagnoses and treatments described below are accurate and in accord with current best clinical practices at the time of writing. However, in view of ever evolving practice patterns with evidence-based medicine, the reader is urged to always evaluate each case and treatment individually at the time of evaluation, and to always consult the most up-to-date sources including drug package inserts, etc., prior to embarking upon treatment.
|
Glossary of Abbreviations (in order of appearance):
|
Case History
|
A 24-year-old woman presents to your office for evaluation. She has just moved to your city. She reports a 10-year history of wearing contact lenses. She first used toric soft lenses (SCLs), for myopia and astigmatism, but decreasing vision then led to the diagnosis of keratoconus, a progressive, bilateral but asymmetric, corneal dystrophy characterized by central corneal thinning, as well as loss of visual acuity through central corneal distortion primarily and corneal scarring secondarily.
|

Contact Lens Systems
Contact lens [CL] systems consist of:
|
|
All these factors potentially participate in complications, which can involve insults from mechanical, desiccation-hydration, hypoxia, toxic, immune-mediated (e.g., allergy) and infectious etiologies.
Given all these interconnected factors, it is impressive that the vast majority of cosmetic CL wearers do so asymptomatically.(1)
Complications, Historically
Fifty years ago, all CLs were "hard," made from oxygen impermeable polymethylmethacrylate (PMMA), and known complications were limited to: injected conjunctivae ("red" eyes); poor comfort (ranging from mild CL awareness to substantial foreign body sensation); corneal edema [then defined by central circular clouding (CCC) and distorted keratometry mires]; peripheral desiccation (so called "3 and 9 o'clock" or "juxtapositional" staining); damaged/soiled CLs; and corneal epithelial abrasion. Microbial corneal infection [microbial keratitis (MK)] was quite rare.(2)
Because only a few symptomatic physiological complications were seen, becoming a good diagnostician was a challenge. CL success could be defined as much by comfort-related tolerance as by physiology-related tolerance.(3) The introduction of SCLs around 1970 improved subjective tolerance and hence prevalence dramatically, but also began the recognition of an array of complications. MK became a particular concern when extended wear became common in the 1980s.
Complications, Today
Clinicians now diagnose a variety of complications. Fortunately, CL wearers rarely experience vision-threatening complications (limited to MK and corneal neovascularization). Table 1 groups the various complications both by their anatomical order and by etiology: mechanical, desiccation-hydration, hypoxia, toxic, immune-mediated and infectious.

Table 1. Contact Lens Complications By Layer and Etiology.
Most complications are primarily caused by CL wear. Some, however, have other etiologies but, by definition, complicate CL wear and deserve attention.
Abbreviations are shown in squared brackets [XX].
| Lids |
|
||||||||||||
| Tear Layer |
|
||||||||||||
| Bulbar Congjunctiva |
|
||||||||||||
| Corneal Epithelium and Anterior Limiting Lamina (Bowman's) |
|
||||||||||||
| Corneal Stroma |
|
||||||||||||
| Corneal Endothelium and Posterior Limiting Lamina (Descemet's) |
|

Forister et al.(1) found that 50% of CL wearers had complications worthy of management but only 1.5% were symptomatic. Few hypoxic complications were noted in this study, primarily corneal neovascularization (which may have been associated with previous low oxygen permeable SCL wear). The most common complications by far were those related to ocular inflammatory issues and allergy, affecting about 30% of subjects. Chalmers et al.(4) found that, among the 18% of CL patients requiring some form of treatment (n=1276) during two years of retrospective evaluation, 10% had lid reactions, 21% had disrupted corneal epithelia, while 14% suffered allergic, 12% conjunctival, 15% inflammatory and 22% infectious (both CL-related and otherwise) reactions. Age (<25 years) and higher myopic refractive error (>5 D) were both associated with greater risk of complications.
Some complications are simple, some multi-factorial and others are collateral — though not directly caused by CLs, these factors "complicate" wear.
Lid Complications
Lid reactions include blepharitis and meibomian gland dysfunction (MGD), which sometimes lead to styes and chalazions; allergic responses, including solution reactions and CL (often "giant") papillary conjunctivitis [CLPC or GPC], and GP lens lid manipulation or GPC-induced upper lid ptosis.(5)(6)(7)
GPC is thought to be a type I Gell/Coombs hypersensitivity response (with elements of type IV) to biological debris on the CL or perhaps to CL mechanical irritation. Secondary sequellae include loss of CL tolerance and ptosis. The patient diagnosed with GPC should first discontinue CL wear until asymptomatic. Signs, such as mucus, inflamed and staining tarsal conjunctival papillae, etc., should subside before CL wear is cautiously resumed with improved cleaning (e.g., increased use of peroxide-based disinfection and/or enzyme cleaner) and/or often more frequent SCL replacement. One-day disposable SCLs are also helpful. It is also useful to change the CL design (e.g., from SCL to GP or vice-versa). Some silicone hydrogels are particularly likely to cause GPC. Topical mast cell-stabilizing agents, NSAIDs, antihistamines and occasionally steroids may be prescribed adjunctively for those patients for whom conservative treatment fails.(8)(9)(10)(11)(12) Steroids should be used with caution, however, to minimize the risk of secondary ocular infection, glaucoma or cataract.

Figure 2. Giant (or Contact Lens-)Papillary Conjunctivitis [GPC or CLPC].
Click image for larger view.

Blepharitis and meibomian gland dysfunction, both infectious and non-infectious, are not caused by CL wear but complicate CL use. Lid treatment includes lid hygiene(13) and occasionally topical antibiotic drops or bedtime ointment use. Occasionally, recalcitrant blepharitis deserves topical steroid/antibiotic treatment (usually discontinuing CL wear) or oral antibiotics.
Hypoxia
Corneal hypoxia was the most common complication of CL wear until the modern era of high oxygen transmissibility CLs.(14)(15)(16)
Cornea hypoxia causes: epithelial microcysts and microcystic edema [MCE]; pseudodendritic edematous corneal formations [ECF]; decreased epithelial mitosis, sensitivity and adhesion; central circular clouding [CCC]; changes in stromal thickness, acidosis and striae; endothelial blebs and polymegethism; corneal distortion/warpage which leads to "spectacle blur."(17)(18)(19)(20)(21)(22)(23)(24)(25)(26) A putative "corneal exhaustion syndrome [CES]" causes previously successful GP CL wearers to become acutely intolerant.(27)
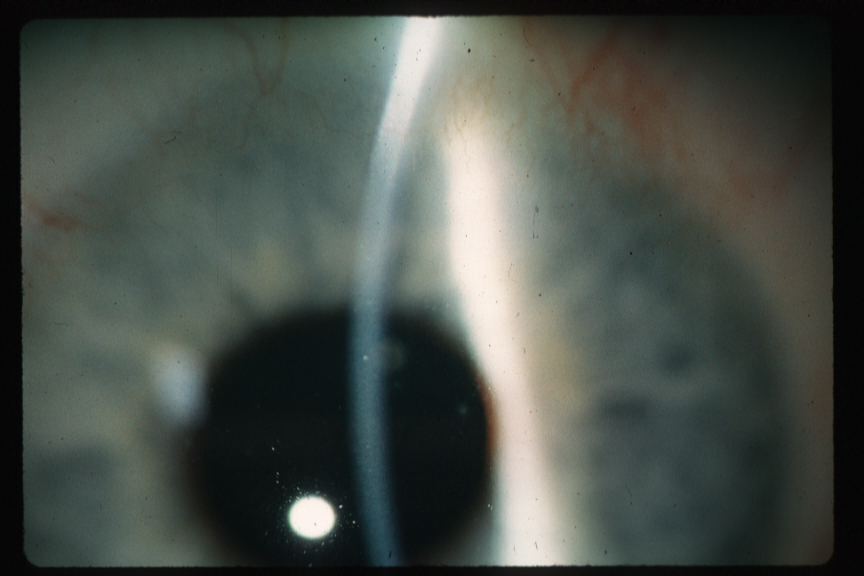
CL hypoxia causes limbal vascular injection.(28) Superficial corneal pannus is associated with SCL hypoxia (typically, superiorly), while mechanical chronic GP CL 3 o’clock and 9 o’clock epithelial desiccation leads to pannus (vascularized limbal keratitis [VLK] or pseudoptyergium) in this location.(29)(30) Deep stromal neovascularization occurs, particularly in keratoconus (see Figures 1A and 1B) but is rare. Secondary intracorneal hemorrhages can also occur with neovascularization
.
Figure 3. Pannus Secondary to GP CL-induced 3 and 9 O'clock Corneal Staining.
Click image for larger view.

It is now clear that maintaining pre-corneal oxygen tension at about 100 mm Hg will preclude clinically significant hypoxia and sequellae. For modern CL, both GP and silicone hydrogel, oxygen permeabilities of 50 to 170 x 10-11 cm/sec ml O2/ml mmHg or greater should maintain the 100 mm Hg level under most daily wear conditions.(31)
If hypoxic signs are noted, the clinician should discontinue any extended wear and/or enhance CL oxygen transmissibility.
Inflammatory (Toxic and Hypersensitivity) and Mechanical Complications
Damaged corneal epithelium stains with sodium fluorescein dye and clinically this is called superficial puntate keratitis or superficial punctuate staining [SPK or SPS]. Causes of SPS include desiccation;(32)(33) chemical-toxic or immune-allergic insult (solution induced corneal staining [SICS]); mechanical/trauma (cracked or chipped CLs, bound or non-moving GP CLs, foreign bodies trapped between the CL and the eye; superior epithelial arcuate lesion [SEAL] also known as "epithelial splitting."(34)
The condition of the patient’s lids/meibomian glands and tear layers can also contribute to SPS. Currently, the most prevalent complications of CL wear are those associated with toxicity, allergy (perhaps environmental or iatrogenic from care solutions) and CL spoilage, often leading to SPS.(1)

Figure 4A. Corneal Epithelial Staining Secondary to Bound GP CL. Note imprint of lens edge.
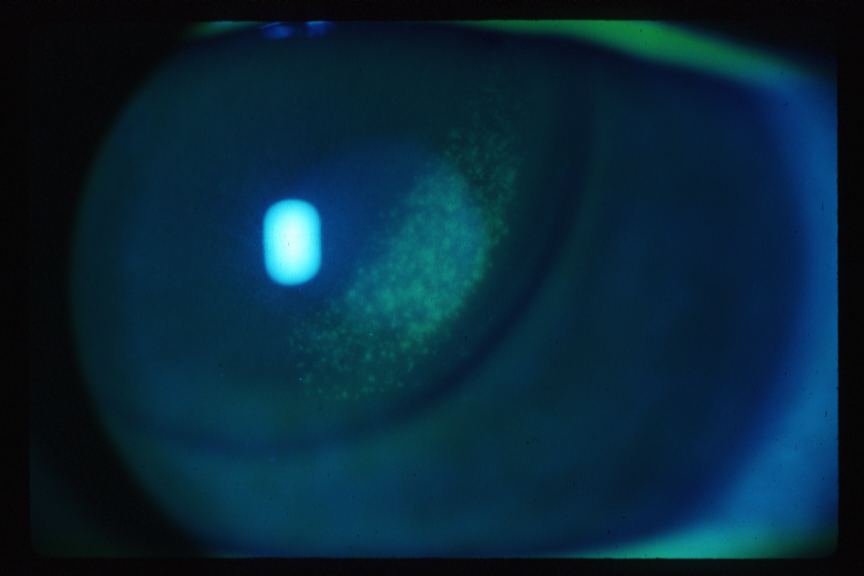
Click image for larger view.

Figure 4B. Corneal Epithelial Abrasion Secondary to GP CL Wear. Note absence of corneal infiltrate.
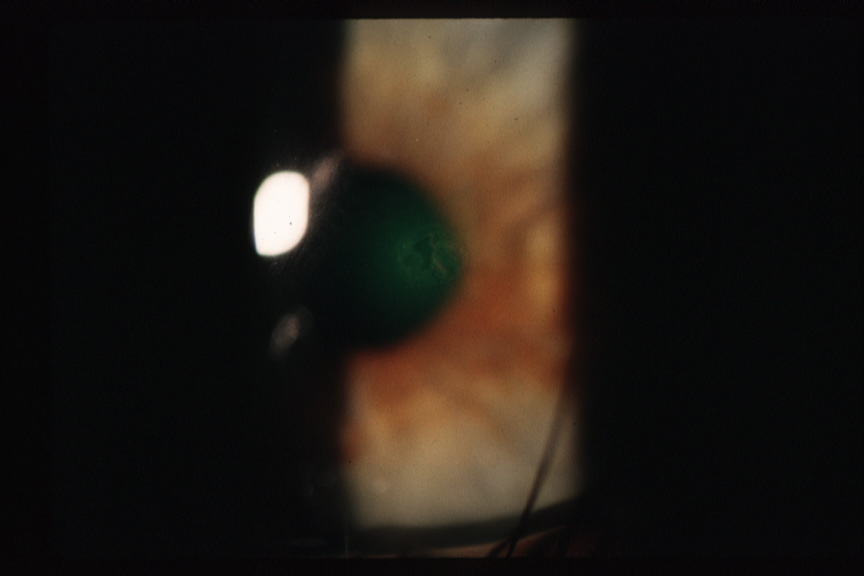
Click image for larger view.

Clinicians must distinguish SPS from less worrisome "dimple veil," wherein round corneal epithelial depressions develop from bubbles of air, or even rolled up balls of mucin, trapped between CLs and the cornea. Flattening GP base and edge curves, as well as artificial tear supplementation, may help manage dimple veil but sometimes (as in the case of keratoconus) this is impractical. Minimal and asymptomatic epithelial dimple stain can be simply monitored. Piggyback CL systems (a rigid lens on top of a SCL) may also prove beneficial in the management of challenging dimple veil.
Patients with SPS commonly present with subjective ocular irritation (itching, dry eye symptoms). Some can be asymptomatic. Other signs include tarsal conjunctival papillae, sub-epithelial infiltrates [SEI], conjunctival injection and/or edema, and even CL superior limbic keratoconjunctivitis.(35)(36)(37) The reader should note that SEIs, both round and dendritic, can also be a sign of corneal infection.
CL solution reactions occasionally can evolve into a devastating destruction of the corneal limbal stem cells. Patients report irritation and the clinician will note a granular, fibrovascular pannus in the involved cornea. Treatment is discontinuation of CL wear, though some patients have required limbal stem cell transplantation.(38)
Goals of acute treatment of SPS and non-infectious SEI are to decrease both subjective patient discomfort and the risk of secondary infection. Treatment of common solution reactions includes discontinuing CL wear, observation, lubricating artificial tears (often unpreserved, unit dose), NSAID and/or anti-histamine drops and. occasionally, in severe cases, topical steroids. The clinician should always rule-out MK before beginning steroid treatment, especially of any SEI. Chronic management of SPS includes modification of both CL design and/or care to preclude re-occurrence. CL wear can be reconsidered with use of an alternative care regimen, often peroxide solutions. SCL wearers may be fitted with daily disposables to eliminate all solution issues. Rigid GP wearers can rinse their CLs with sterile preservative-free saline prior to insertion.
CL soilage (surface deposits, films and debris) and damage (cracks, scratches, chips) can result in similar signs/symptoms. SCLs are now usually "disposable" (1-day, 2-week, 1-month or 3-month) and therefore can be replaced before they get soiled. GP CLs can be reconditioned by polishing and cleaning but eventually they become warped, scratched or soiled and should be replaced.
Contact lens acute red eyes [CLARE] primarily occur during extended wear and SEIs may be in the clinical picture. These reactions are believed to be associated with bacterial contamination of CLs and associated solutions, cases, etc., leading to ocular exposure with bacterial toxins. Initial treatment is palliative, and improved CL care and hygiene — and discontinuing extended wear — should preclude this complication.
Corneal Abrasion
When abrasion(39)(40) is diagnosed, the clinician must rule out and prevent infection, as well as manage symptoms (pain primarily). Abrasion can be due to lens defects (chips, cracks), flat fitting lenses, tight edges, bound lenses, foreign bodies trapped under lenses, underlying etiologies of keratoconus or epithelial basement membrane dystrophy, etc. Patients should temporarily discontinue CL wear to aid the healing process. Some clinicians prescribe prophylactic antibiotic treatment, while others prefer to withhold antibiotics unless infection is present. To decrease the risk of precipitating or enhancing MK, the clinician should refrain from eye patching and topical steroids.(41) Often, lubrication with artificial tears and professional supervision (follow-up at 24 h or immediately if symptoms increase) is appropriate treatment. Close professional supervision should continue until the epithelial defect has closed. The clinician should also address the cause of the abrasion in order to preclude additional events.
Microbial Corneal Infection [MK]
Microbial keratitis [MK] has symptoms of foreign body sensation or ocular pain, photophobia, "red" eye, discharge, and clinical signs of a corneal epithelial/stromal defect with associated inflammatory response (SEI); solitary, large, painful lesions are more worrisome than small, multiple, peripheral lesions.(42) MK is often accompanied by anterior chamber reaction (including hypopyon in some cases), ocular discharge, lid swelling and conjunctival injection. MK is commonly unilateral.
MK is clearly associated with CL extended wear — a robust incidence has been found at ~20 per 10,000 people/year using SCLs for extended-wear across a wide range of studies (and ~4 per 10,000 people/year using SCLs for daily-wear; MK incidence is probably lower with rigid GP CLs).(43)(44)(45)(46)(47)
Other risk factors include poor CL care and hygiene (e.g., "topping off" rather than changing solutions in lens case, dirty cases, etc.), travel, smoking, male gender and young adult age, and compromised immune systems.(48)(49) Overnight orthokeratology may be an additional risk factor.
Because MK is a sight-threatening disease, suspicious lesions should always be presumed to be infectious and treated aggressively. Whenever any of the signs or symptoms of MK occur, CL wear should be immediately discontinued in both eyes to decrease the potential for bilateral disease.
Bacterial MK is usually attributable to Gram-negative Pseudomonas aeruginosa, but also Gram-positive Staphylococcus aureus and Staphylococcus epidermidis. It has been primarily associated with CL wear extended through one or more sleep cycles.(50) Poor compliance with appropriate CL care such as exposing CLs to fresh water (e.g., wear while swimming) also appears to be a major risk factor for bacterial infection as well as Acanthamoeba MK(51) during daily wear. Fungal infection has been associated with compromised ocular surfaces and topical steroid use in addition to poor CL care, especially "topping-off" rather than exchanging solutions and cleaning cases.(52)
Management of MK begins with initial timely recognition. Clinicians at hospitals and university medical centers usually obtain cultures and smears of all suspicious lesions. Community doctors alternatively often treat peripheral and small suspected MK empirically.(53) Treatment of suspected bacterial MK begins with an initial "loading" dose using antibiotic drops every fifteen minutes for the first hour or two of treatment, followed by additional drops every hour while the patient is awake. Many clinicians believe 4th generation fluoroquinolone monotherapy (off-label use, as these agents are only FDA-approved for treatment of conjunctivitis) is as effective as dual therapy with "fortified" aminoglycosides (e.g., Gentamicin, Tobramycin, Amikacin) plus a cephalosporin or Vancomycin, particularly for small and peripheral suspected bacterial infections. Professional supervision should be frequent, often at 24 h intervals if not sooner.
Treatment is modified by the patient’s clinical progress, as well as the laboratory identification of microorganism(s) and antibiotic sensitivities. Adjunctive patching should be avoided. The early use of topical steroids is usually contraindicated but some doctors intervene with steroids in order to limit scar formation. Steroid treatment, however, may allow inadequately controlled infections of Pseudomona sp, herpes and Acanthamoeba to escape therapy.
The clinician should always consider the possibility of fungal, herpetic, mycobacterial and Acanthamoeba infection in any CL MK, especially in cases of chronic disease with initially negative culture results and failure to respond to antibiotic therapy. Clinical suspicion of Acanthamoeba should be increased when the patient reports extreme ocular pain or when an unusual epitheliopathy (reminiscent of herpetic epithelial disease) or peripheral corneal radial neuropathy are observed. Special techniques help diagnose Acanthamoeba MK (e.g., confocal microscopy or tissue biopsy). Medical treatment of Acanthamoeba keratitis often employs combinations of antibiotic, antifungal, antiparasitic and biocide/cationic antiseptic agents.(54)
Fungal MK must also be considered for which antifungal pharmaceutical agents (e.g., Natamycin, Voriconazole) (both commercial and custom-made) are available. It is important to note that herpetic, atypical mycobacterium and Acanthamoeba infections often mimic fungal corneal ulcers and vice-versa. Misdiagnosis and medical failures are common when treating MK caused by these microorganisms.
Adenoviral and herpes viral corneal infections can occur during CL wear. No causative association has been proposed for such viral infections. CL wear should be discontinued, however, during viral infections.
CLs that have been used during (the early stages of) MK should probably be discarded and, if CL wear is to be continued, new CLs dispensed once the infection has resolved.
MK, while rare, remains a major concern and management is complex. Aggressive medical treatment including subconjunctival injections and/or systemic antibiotic treatment with hospitalization, and perhaps corneal transplantation, may be necessary, especially in cases of indolent, refractory or non-bacterial corneal infections. Hallmarks of successful treatment/healing include improved patient comfort (i.e., decreasing pain), reduced inflammatory signs and closing of epithelial defects. It is a good idea to refer patients with severe or refractory inflammatory or infectious ocular disease to a corneal and external eye disease specialist.
General Treatment Comments
The most effective way to address the varied complications of CL wear detailed above is to prevent them from occurring. Corneal hypoxia is very rare with modern lenses during daily wear. Restricting CL use to daily wear should also reduce the occurrence of bacterial MK. Many complications can also be avoided by optimal CL care and hygiene (e.g., refraining from: overwear of CLs,(56) use of tap water in any aspect of CL care, re-using CL solutions and/or soiled cases, etc.) consistent with both common sense and FDA-approved manufacturers’ guidelines.
CL patients should understand their role in their own protection. Patients should present at reasonable intervals for routine professional care so that mild asymptomatic complications can be diagnosed and addressed and the use of care solutions monitored and adjusted.(1) Symptomatic patients should receive appropriate evaluation and treatment in a timely manner, probably within 12 h of the onset of symptoms.
It is well to keep in mind that the vast majority of CL wearers rarely experience severe complications, while they substantially benefit from the optical and cosmetic advantages of modern contact lenses and care.