Course Authors
Robert J. Pignolo, M.D., Ph.D.
Dr. Pignolo is Assistant Professor and Director, Ralston-Penn Clinic for Osteoporosis & Related Bone Disorders, Department of Medicine, Division of Geriatric Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA.
Within the past 12 months, Dr. Pignolo reports no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the presenting signs and symptoms in Paget's disease of bone
Discuss the most likely etiologies for Paget's disease of bone
Summarize and apply the major treatment options for patients with Paget's disease of bone
Define possible complications of Paget's disease of bone.
Paget’s disease of bone (osteitis deformans) is a disorder of bone metabolism where accelerated bone remodeling at focal locations results in bony overgrowth and impaired bone microarchitecture. First described in 1877 by Sir James Paget, Paget’s disease is primarily a condition whereby excessive osteoclastic bone resorption is followed by excessive bone formation. In asymptomatic presentations, diagnosis is made by elevated bone serum alkaline phosphatase or by incidental findings of plain radiographs obtained for other reasons. Symptomatic patients present with pain and skeletal deformities secondary to overgrowth of affected bone. It is the second most common bone disease after osteoporosis.
Epidemiology
The prevalence of Paget’s disease is estimated to be as high as 3.7 percent of individuals over the age of 40 years,(1)(2) increases with age and affects men and women almost equally.(2) Paget’s disease is more common in populations of Anglo-Saxon origin (United Kingdom, Australia, New Zealand, North America and Western Europe), and is rare in Asia, India and Scandinavia. About one percent of the population in the United States over the age of 40 years is affected.
Etiologies
Genetic and environmental factors (i.e., infectious disease) may likely play causative roles in the pathogenesis of Paget’s disease. A clear etiology, except perhaps in familial forms, is lacking.
Up to one-quarter of family members of patients with Paget’s disease eventually contract the disease,(3)(4)(5) with first-degree relatives having a seven- to ten-fold increase in the likelihood of developing the condition.(5)(6) Susceptibility loci have been identified in families with apparent autosomal dominant inheritance.(7)(8)(9) In those with familial forms, mutations in the gene for sequestosome 1 (SQSTM1) have been found in some affected patients.(6) Sequestosome 1 encodes a ubiquitin binding protein that acts as an anchor protein and is involved in NF-κB signaling. Some patients with Paget’s disease who have inclusion body myopathy share mutations in the gene for valosin-containing protein with those who suffer from frontotemporal dementia.(10)
Viral infection has also been implicated in the etiology of Paget’s disease. Osteoclasts from patients with Paget’s disease appear to contain viral particles, possibly a member of the Paramyxoviridae family, which are not found in osteoclasts from unaffected individuals.(11)(12)(13)(14) In a small number of patients with Paget’s disease, osteoclast precursors and peripheral blood mononuclear cells harbored viral transcripts that implicate the measles virus.(15) Although a relationship between Paget’s disease and the canine distemper virus has been suggested in the owners of unvaccinated dogs, this possibility is controversial.(16)(17)
Histologically, the characteristic feature of Paget’s disease is disorganized lamellar bone due to marked bone resorption by numerous large osteoclasts, followed by a compensatory acceleration in bone formation. The marrow spaces of this mosaic trabecular bone are filled by excess fibrous tissue with marked hypervascularity.(18)(19) The hallmark of active lesions is the presence of hyper-multinucleated osteoclasts adjacent to many osteoblasts which synthesize bony matrix so quickly that collagen produces an immature woven pattern. Features of late stage lesions are thick trabeculae with prominent cement lines at the interfaces of numerous previous repetitions of bone resorption followed by bone formation. Sclerotic bone may result after resolution of hypercellular lesions. This abnormal skeletal remodeling begins at one end of a bone and slowly but progressively advances to affect the entire bone. Fronts of osteolytic activity progress at the rate of about one centimeter per year. Cortical thickening (hyperostosis), disorganized course trabeculae (osteosclerosis) and bony expansion precede so-called "burnt out" disease where bones are widened and nonuniformly ossified.
Clinical Manifestations
Most patients are asymptomatic at the time of diagnosis and Paget’s disease is suspected by an elevated serum alkaline phosphatase or by suggestive findings on a plain radiograph obtained for other reasons.
Paget’s disease may involve either a single bone (monostotic) or a few bones (polyostotic) but the process does not spontaneously spread to adjacent bones. In symptomatic patients, pain and skeletal deformities usually suggest the disease. In more advanced disease, fractures, osseous tumors, neurologic findings, cardiac disease and calcium or phosphate imbalances may be apparent with the initial presentation. Less commonly, unexplained or excessive bleeding during orthopaedic surgery may be the first signs of Paget’s disease.
Variability in signs and symptoms relates to the degree and location of bone lesions, and the most common sites of skeletal involvement are the pelvis, spine, skull and long bones. Pain may occur at the site of an active pagetic lesion or in areas where pagetic complications arise secondary to degenerative arthritis, nerve impingement syndromes or osteosarcoma. Bone pain may persist throughout the day, be present at rest and may worsen upon weight bearing, but these are non-specific symptoms. The likelihood of damage to joints adjacent to bony deformities is high, and osteoarthritis as a source of pain can develop in the knees and hips. Figure 1 depicts possible etiologies for pain at active pagetic lesions or due to complications.

Figure 1. Possible Etiologies for Pain Associated with Paget’s Disease of Bone.
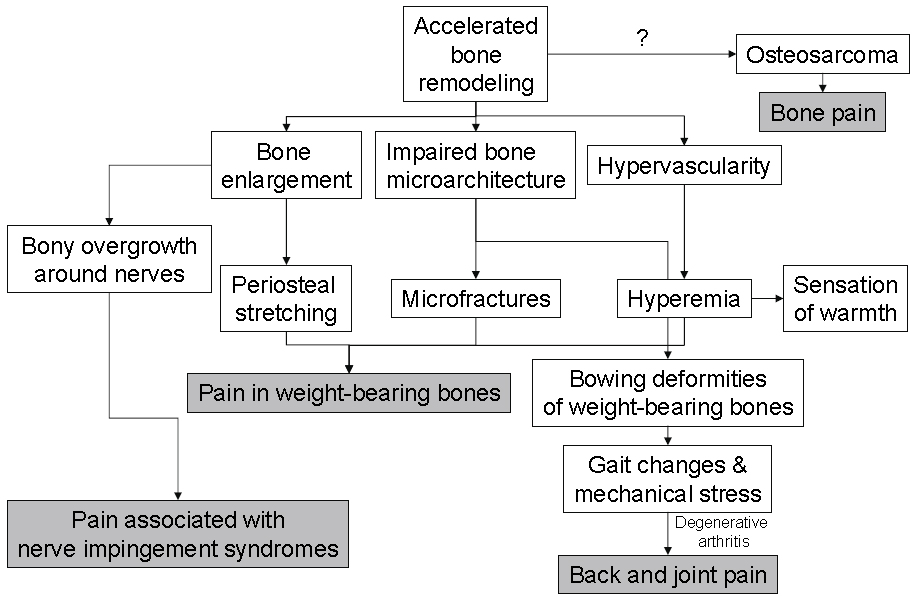
Click image for larger view.

Skeletal overgrowth or deformities may be apparent when the disease involves the skull, jaw, clavicle or long bones of the lower extremities. Skeletal deformities commonly occur in the long bones, skull and clavicles (Figure 2). Bowing in the tibia and femur may precipitate gait abnormalities and cause mechanical stress and pain. Hyperemia in affected locations may also cause perceived and/or objective warmth. Prominent dilations of the superficial scalp veins may also be apparent.

Figure 2. Common Deformities of the Skull and Weight-bearing Long Bones.
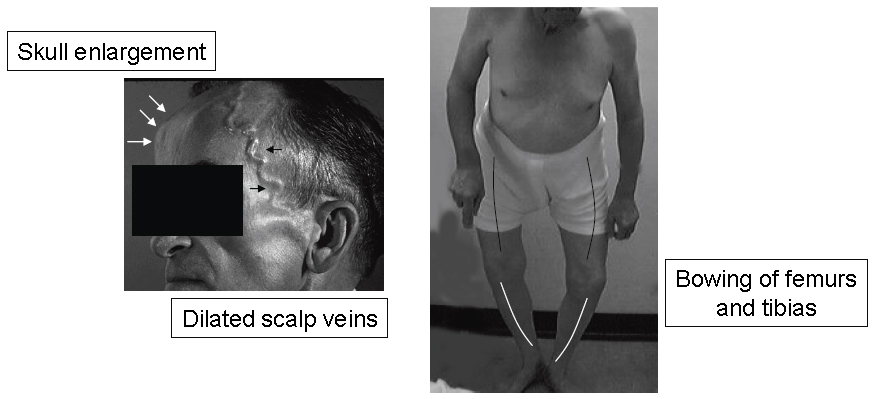
Click image for larger view.

Paget’s disease can be identified by plain radiographs. Typical findings on x-rays of the long bones may include a characteristic osteolytic front that forms a "blade of grass" sign, bony expansion, thickened cortices, and areas of osteosclerosis and osteopenia in a patchwork design. Many of these characteristic radiographic features can be viewed at http://www.paget.org.uk/info/tests.htm#xray. Inconspicuous skull involvement (osteoporosis circumscripta, or radiolucent areas on skull films) progresses over time to overt skull enlargement with diffuse sclerotic changes, typically in the frontal and occipital regions (so-called "cotton-wool" skull on plain radiographs). Cortical thickening and lytic lesions in the spine give rise to "picture frame" vertebral bodies by x-ray.
Traumatic and spontaneous fractures occur commonly in patients with Paget’s disease. Femoral or tibial fractures can be either complete or incomplete, with protracted acute blood loss a complicating issue in the case of complete fractures. Although acute fractures through active lesions can heal rapidly, fracture malunion is not uncommon in the proximal femur.(20)
Bone tumors are increased in patients with Paget’s disease, and can be associated with new or increased pain and possible masses at pagetic sites. Osteosarcomas are the most common tumors and have a poor prognosis. Although they develop in less than one percent of patients with Paget’s disease, these osteosarcomas are more aggressive than in age-matched individuals.(21) Complications and ultimately death typically result from local extension or pulmonary metastases. Benign giant cell tumors also occur, usually involving the skull and facial bones.
Neurologic complications (Table 1) are caused by bony impingement of nerves or disruption of the blood supply.(22)(23)(24)(25) Compressive nerve entrapment (e.g., hearing loss due to pagetic skull involvement of the 8th cranial nerve) and other cranial nerve deficits occur but are not common. Hearing loss, when it occurs, may be related to temporal bone involvement, is often bilateral and progressive, and can present as high-frequency sensory loss and low-frequency conductive loss. Hydrocephalus with headache and dizziness is rare. A report of greater prevalence of depression in patients with Paget’s disease may be unfounded.(26)(27)

Table 1. Neurologic Complications in Paget’s Disease of Bone.
| Complication | Etiology |
|---|---|
| Hearing loss | 8th cranial nerve compression or involvement of the middle ear ossicles |
| Visual disturbance and facial palsy | 2nd, 5th and 7th cranial nerve compression |
| Invagination of the skull by cervical vertebrae (platybasia) | Involvement of the base of the skull |
| Hydrocephalus | Blockage of the aqueduct of Sylvius |
| Radicular neuropathies, spinal stenosis | Nerve impingement or ischemia due to "vascular steal" syndrome |
| Myelopathy, ischemic myelitis | Nerve impingement or ischemia due to "vascular steal" syndrome |
| Mottled retinal degeneration; angioid streaks | Unknown |

Cardiac abnormalities are related to the severity of Paget’s disease as assessed by involvement of at least three major bones that are at least 75 percent affected. Cardiac complications include high output heart failure (rare), calcific aortic stenosis and conduction abnormalities (complete and incomplete atrioventricular block and bundle branch block).(28) Nephrolithiasis and Peyronie’s disease have also been associated with Paget’s disease.(29)
Although calcium and phosphorus concentrations are usually normal in patients with Paget’s disease, hypercalcemia and hypercalciuria may occur with immobilization or fracture. However, hypercalcemia in an ambulatory patient likely indicates a second disorder such as primary hyperparathyroidism.(30)(31) Secondary hyperparathyroidism can also be induced by hypocalcemia in patients with active Paget’s disease due to a marked increase in calcium requirement unmet by adequate intake(32) or by suppression of bone resporption with bisphosphonates while bone formation continues.(33)
Biomarkers that correlate with bone resorption, such as urinary hydroxyproline, as well as those that reflect bone formation (e.g., serum alkaline phosphatase) are useful to monitor activity, disease severity and treatment efficacy. They are usually elevated in patients with polyostotic disease and normal in patients with monostotic disease; both decrease with successful treatment. Other markers of bone metabolism may be less useful.(34)
Diagnosis
After a careful history and physical examination, measurement of the serum concentrations of alkaline phosphatase and calcium should be undertaken. A high serum alkaline phosphatase level should raise high clinical suspicion for Paget’s disease in an otherwise healthy older patient with a normal serum calcium and no evidence of liver abnormalities or osteomalacia.(29)(35) Levels of alkaline phosphatase can be normal when there is just a small focus of active Paget’s disease or after medical treatment of co-existing conditions that result in decreased bone turnover (e.g., treatment of osteoporosis with a bisphosphonate).
Focal areas of increased uptake or "hot" spots" on radionuclide bone scan is a sensitive test for identifying pagetic bone lesions. Plain radiographs can give additional information with respect to adjacent joints, fissure fractures, lytic areas and bone deformities.(36) Areas of enhanced radionuclide uptake reflect increased bone formation and blood flow, and may be identified before the appearance of any radiographic changes. Mature or "burnt-out" lesions may not appear on bone scan since pagetic activity has ceased. In polyostotic Paget’s disease, skeletal scintigrams can show focally enhanced radionuclide uptake with partial as well as complete bone involvement.
Pagetic lesions on bone scans need to be distinguished from metastatic bone disease, and if not possible, further studies should be considered. If malignancy is suspected, computed tomography, magnetic resonance imaging or bone biopsy should be considered. However, a bone biopsy is rarely needed and increases the risk of fracture if performed on weight-bearing lesions.(29)
Treatment
The majority of patients with Paget's disease of bone are asymptomatic, and they are often not treated. Although some asymptomatic patients may benefit from treatment, the primary indications for pharmacologic intervention are to relieve symptoms (pain, warmth) and to treat or prevent complications associated with the disease.(37)(38)(39) Asymptomatic patients with involvement of weight-bearing long bones, pelvis, skull or spine should be treated to avoid potential progression to deformities and complications.
Clinical and biochemical indications for treatment include bone aches and pain, headache due to skull involvement, hypercalcemia from immobilization and symptoms of nerve compression.(37)(38)(39) Bowed extremities will not change. Deafness is unlikely to improve. Pain from secondary arthritis may or may not improve, but when both Paget’s disease and osteoarthritis are present, the arthritis may be treated first with acetaminophen or a nonsteroidal antiinflammatory drug.(40)(41)
Prophylactic therapy is also used in patients before elective surgery on the affected bone(s) to reduce the hypervascularity associated with potentially excessive blood loss.(42)(43)(44) The rare development of hypercalcemia in association with immobilization in patients with polyostotic disease also warrants prophylactic treatment.
Major treatment modalities in Paget’s disease include non-pharmacological therapy (physical therapy to improve muscle strength and mobility, and to control some types of pain), pharmacological therapy using either bisphosphonates or calcitonins,(45)(46)(47)(48)(49)(50) pain management using analgesics, and surgery. Shoe inserts, orthotics and assistive devices for ambulation, in addition to physical therapy, may be useful in addressing gait abnormalities or other difficulties resulting from bowing of the lower extremities. Counseling of patients with respect to fall prevention and fractures (including care to avoid heavy lifting, sudden or strenuous twisting, turning or flexion movements in those with vertebral involvement), as well as weight control in overweight patients (to minimize pain exacerbated by weight-bearing) may be helpful. Pharmacologic and surgical interventions will be discussed below.
All drugs used to treat Paget's disease suppress osteoclastic activity and are listed in Table 2 with their typical dosing regimen. Side effects due to calcitonin include nausea (~10%), local irritation at injection site (10%), flushed ears and face (10-20%), bronchospasm (rare), uriticaria (rare) and anaphylaxis (rare). Secondary drug resistance occurs in greater than 50% of patients treated for more than 6 months due to development of calcitonin antibodies, with higher titers usually producing greater resistance.

Table 2. FDA-Approved Bisphosphonates for the Treatment of Paget's Disease of Bone.
| Zoledronic Acid Trade Name: Reclast®(Novartis) |
5 mg intravenous infusion over 15 minutes. (Suppression of disease activity <e; 2 years) |
|
Risedronate Trade Name: Actonel®(Procter & Gamble/Aventis) |
30 mg once daily for 2 months |
| Tiludronate Trade Name: Skelid®(Sanofi-Synthelabo, Inc.) |
400 mg (two 200 mg tablets) once daily for 3 months |
| Alendronate Trade Name: Fosamax®(Merck) |
40 mg once daily for 6 months |
| Pamidronate Trade Name: Aredia®(Novartis) |
30 mg intravenous infusion over 4 hours on 3 consecutive days or 60 mg or 90 mg intravenous infusion over 2-4 hours, repeated as clinically indicated |
| Etidronate Trade Name: Didronel®(Procter & Gamble) |
200 to 400 mg once daily for 6 months |
| Cacitonin Trade Name: Miacalcin®(Novartis) |
Injection, 50 -100 units daily or 3 times per week for 6-18 months |

Adverse effects from bisphosphonates include upper gastrointestinal intolerance, diarrhea with oral forms, acute phase reaction, musculoskeletal/bone pain, hypocalcemia, renal failure (with high dose intravenous bisphosphonates), ocular inflammation (rare) and osteonecrosis of jaw (rare). Contraindications to bisphosphonate use include hypocalcemia, vitamin D deficiency, hypoparathyroidism and severe renal insufficiency.
Elective surgeries such as joint replacement, tibial osteotomy or internal fixation of fractures may benefit patients with difficult to control pain symptoms. Corrective osteotomy for deformity is usually considered when malalignment causes mechanical overload, limitation of motion, pain and/or dysmorphic appearance. Although hip arthroplasty in Paget’s disease poses difficult surgical technical challenges, they are performed in cases of gross deformities (coxa vara, femoral bowing, acetabular protrusion), as well as for stress fractures of the proximal femur, and subcapital fractures or nonunion. Pretreatment with antipagetic therapy for at least two months prior to elective should be attempted.(42)(43)(44)
Normal serum levels of calcium, phosphorus and 25-hydroxyvitamin D (calcidiol) should be present when initiating bisphosphonate therapy. The newer nitrogen-containing bisphosphonates are effective, relatively safe and appear to have advantages compared to the simple bisphosphonates or calcitonin.(45)(51) The use of calcitonin has largely been supplanted by treatment with bisphosphonates, although it still remains an option for patients who have contraindications to bisphosphonates or in whom they are poorly tolerated. Effective therapy results in a reduction in bone turnover and a substantial fall in serum alkaline phosphatase concentrations. There is no cure.
In situations where Paget’s disease is particularly active or extensive, higher doses or more frequent dosing intervals of a bisphosphonate may be required. Acquired resistance to a specific compound may also occur, but use of an alternative bisphosphonate may overcome initial resistance.(52)
Monitoring
Normalization of serum alkaline phosphatase is associated with biochemical and histological remission; conversely, increases in serum alkaline phosphatase parallel increases in disease activity.(26)(53)(54)(55)(56)(57)(58) Serum alkaline phosphatase can thus be used as an accurate outcome measure when monitoring the response to bisphosphonate therapy.
Serum alkaline phosphatase should be measured at three to six months after therapy to assess the initial response, and once stable can be measured once or twice a year as a marker of bone activity.(51) There is no evidence that other markers of bone turnover are better measures of clinical response.(59) Evidence of increased or recurrent bone turnover by regular measurement of serum alkaline phosphatase, radiographic progression of disease and/or recurrent pain is an indication for re-treatment. New symptoms should be distinguished from secondary or unrelated conditions that are not from current Paget’s disease activity.
Additional Information for Physicians and Patients
The Paget’s Association was founded in 1973 by Anne Stansfield MBE, who was driven by a lack of understanding and interest in the treatment of her husband who had Paget’s disease (http://www.paget.org.uk/). The Paget Foundation was founded in 1978 as a national voluntary health agency located in New York, NY, to provide information on Paget's disease of bone to patients, family members and health professionals (http://www.paget.org/).