Early-onset Parkinson's Disease
Course Authors
Roy N. Alcalay, M.D., M.Sc., and Karen Marder, M.D., M.P.H.
Dr. Alcalay is Assistant Professor of Neurology, and Dr. Marder is Professor of Neurology (in the Sergievsky Center, Taub Institute and Psychiatry), Columbia University College of Physicians and Surgeons, New York.
Within the past 12 months, Dr. Alcalay reports no commercial conflicts of interest and Dr. Marder has received grant/research support from Neurogen.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Identify genetic risk factors for Early-onset Parkinson's disease (EOPD)
List the clinical characteristics of EOPD and apply them to the differential diagnosis of EOPD
Compare EOPD and late-onset PD
Discuss treatment options for EOPD.
Parkinson’s disease (PD) is the second most common neurodegenerative disease after Alzheimer’s disease, affecting up to 1% of the U.S. population older than 65.(1) PD is also rapidly increasing in developing countries.(2) Early-onset PD (EOPD) is less common than late-onset PD. It is estimated that 3-10% of PD cases have an age-at-onset of 40 or younger.(3) The definition of EOPD versus late-onset PD varies in different studies but most define EOPD as first presentation of motor symptoms before the age of 40 or 50.(3)(4)(5) While there are many similarities between EOPD and late-onset PD, EOPD has its unique features. In this Cyberounds® we will review the clinical information known about EOPD. We will divide our review into three sections: etiology, evaluation, and clinical course and treatment.
Etiology of EOPD
Smoking and caffeine consumption confer a decreased risk of EOPD (protective).
The etiology of EOPD, like PD in general, is largely unknown. EOPD is considered a complex disorder and is most likely associated with the effects of multiple genes in combination with environmental factors. Many environmental factors have been linked with PD: the most studied include pesticide exposure, caffeine intake and smoking.(6) Smoking and caffeine consumption confer a decreased risk of EOPD (protective); however, the mechanism behind this association is unknown.(7) Whether reduced smoking and caffeine consumption are secondary to premorbid personality traits (less addictive behavior) or whether specific genetic factors mediate this behavior is unknown.(7)
The role of prenatal and early life exposures in the pathogenesis of PD has not been well defined.(8)(9) Ever since the first causative mutation in α-synuclein (SNCA) was identified in 1997,(10) a wealth of information with regard to the genetics of PD has been accumulated. Several genes and chromosomal loci have been linked with PD and have been designated PARK1 - PARK14. Mutation carriers often develop PD earlier than non-carriers [except for Leucine-Rich Repeat Kinase 2 (LRRK2) carriers].
Frequently, the genes associated with PD are classified as autosomal dominant and autosomal recessive; however, these traditional genetic definitions may not apply. For example, many of the recessive genetic mutations may increase the risk of EOPD even when only a single copy is inherited (e.g., PRKN, DJ-1(11) and PINK-1).(12) The distinction between an autosomal dominant genetic mutation with incomplete penetrance (e.g., LRRK2(13) and α-synuclein(14)) and a susceptibility gene [e.g., glucocerebrosidase (GBA)] is not well defined. Table 1 describes the genetic mutations associated with EOPD, the populations in which the genetic mutations have been described and the clinical course which was reported in the carriers.
GBA mutations may be the most frequent genetic risk factor for PD in selected populations.

Table 1. Selected Genetic Mutations Associated with EOPD.
| Name | Locus | Gene | Original report | At risk populations | Mode of inheritance | Clinical features |
|---|---|---|---|---|---|---|
| PARK1 | 4q21.3 | α -synuclein (a major component of Lewy bodies,(94) the pathological hallmark of PD) |
1997. A large family of Italian descent with multiple affected individuals (mean age- at- onset 46)(10) | Extremely rare. Reported in families from Germany, Italy, United States, Greece, Spain, Korea | Autosomal dominant (point mutations in α-synuclein). |
Similar to idiopathic PD, but carriers may also develop dementia. (14) |
| PARK2 | 6q25.2 - q27 | Parkin | initially discovered in 1998.21 mutations were initially identified in familial cases of EOPD(20) | The most common genetic risk factor for EOPD.(21)(22) Frequency of mutation carriers increases with younger age- at- onset24 ubiquitous, 22 but may be higher in Hispanics24 |
Autosomal recessive. Role of a single mutation controversial (12)(25) (29)(30) (31)(32) (33)(34) (35)(36) (37)(38) (39)(40) (41) |
EOPD, usually slowly progressive and may require less levodopa treatment. (25) Can respond well to deep brain stimulation. (95) |
| PARK4 | 4q | α -synuclein (similar to PARK1) |
American and European families | Autosomal dominant (duplications or triplications in the gene). Incomplete penetrance (33% in one report.(14)) |
Early onset; often accompanied by dementia. Carriers of tripli-cations may be more likely to develop dementia than carriers of dupli-cations (96) |
|
| PARK5 | 4p14 | UCHL1 | Single report of a German family(97) | Two siblings with age- at- onset 49 and 51 | Indistin- guishable from idiopathic PD | |
| PARK6 | 1p35 - p36 | PTEN induced kinase-1 (PINK-1) | Initially described in three con- sanguin- eous European families in 2001. (42)(98) | Mutations are ubiquitous, however, very rare (48)(99) (100)(101) (102)(103) (104)(105) (106) | Autosomal recessive. Role of a single mutation controversial | Slowly progressive, with early onset of drug induced dyskinesias, similar to PRKN. (42)(43) May be associated with psychiatric symptoms including affective and delusional disorders. (107) may benefit from DBS95 |
| PARK7 | 1P36 | DJ-1 | Discovered in 2003 in two European families. (108) | Extremely rare. (109)(110) Described in families in the Netherlands, Italy, Uruguay |
Autosomal recessive | Similar to idiopathic PD |
| PARK8 | 12p11.2 - q13.1 | Leucine-Rich Repeat Kinase 2 (LRRK2) | Discovered in 2004(111) | Ubiquitous, most common in North African Arab and Ashkenazi Jews. | Autosmal dominant (incomplete penetrance) | May present as EOPD or late-onset PD. presentation may be hetero-geneous, (112)(113)(114) but good response to treatment with levodopa and dopamine agonists is often reported. Treatment may be complicated by dyskinesia. (53) |
| PARK9 | 1p36 | ATP13A2 | Identified in 2006 in con- sanguin- eous Jordanian families.(64) | Jordan | Autosomal recessive | Childhood onset of parkinson- ism, dystonia, spasticity. Abnormal MRI |
| Gluco-cerebro-sidase | 1q21 | Gluco-cerebro-sidase (GBA) | Ubiquitous. Common in Ashkenazi Jews | susceptibility gene | Similar to idiopathic PD; higher frequency of self reported cognitive impairment |

EOPD frequently appears with dystonia or stiffness (rigidity) and can be misdiagnosed.
PARK-1, PARK-4: α-synuclein (SNCA)
Mutations in SNCA were the first genetic risk of PD to be identified.(10) Genetic alterations which cause a gain of function, including point mutations (PARK-1), duplications and triplications (PARK-4), have all been found in association with EOPD.(14)(15) In addition to gain of function mutations, Genome Wide Association Studies (GWAS) have implicated common single nucleotide polymorphisms (SNP) in SNCA in the pathogenesis of PD;(16)(17)(18)(19) However, the association of these SNPs association with PD is much weaker than the association with pathogenic mutations.
PARK-2: Parkin (PRKN)
Mutations in the PRKN gene are the most common genetic risk factors for EOPD.(20)(21)(22) While over 100 mutations have been identified in the PRKN gene, their frequency in non-familial samples is lower than previously reported.(23) PRKN mutation carriers usually have a younger age-at-onset than non-cariers,(24) require lower doses of levodopa therapy and may present with dystonia; however, one study suggested that dystonia is associated with the early age-at-onset rather than mutation status.(25) Carriers of two PRKN mutations may further differ from idiopathic EOPD in their preserved sense of smell,(26) and the lack of Lewy bodies on autopsy.(27) The presence of Lewy bodies in the substantia nigra in the midbrain is the pathological hallmark of PD. Of the six autopsies of mutation carriers which have been described, only two had Lewy bodies.(28)
The role of a single PRKN mutation in the pathogenesis of EOPD is controversial.(12)(25)(29)(30)(31)(32)(33)(34)(35)(36)(37)(38)(39)(40)(41) In one large EOPD genetic study, there were more heterozygote PRKN mutation carriers than homozygotes/compound heterozygotes;(23) carriers of a single PRKN mutation often develop EOPD with a later age-at-onset than carriers of two mutations.(24)
PARK-6: PTEN induced kinase-1 (PINK-1)
The natural history of PINK-1 related EOPD may be very similar to that of PRKN related EOPD.(42)(43) As in PRKN, carriers of mutations on both alleles, either homozygotes or compound heterozygotes, develop EOPD. Similar to PRKN, the role of heterozygous PINK-1carriers is controversial.(44)(45)(46)(47)(48)(49)(50) However, in contrast to PRKN, PINK-1 related EOPD presents with impaired olfaction(51) and a recent report of a single autopsy reported Lewy body pathology.(52)
PARK-8: Leucine-Rich Repeat Kinase 2 (LRRK2)
LRRK2 mutations are the most frequently identified genetic mutations in late-onset PD.(53) In contrast to the other PARK mutations, mutation frequency may be similar in late-onset PD and EOPD.(53)(54) A single study of 953 EOPD individuals reported that LRRK2 is the third most common genetic risk factor for EOPD after PRKN and GBA.(23) Different mutations have been identified in LRRK2, and as with SNCA, pathogenic mutations are probably gain of function mutations.
Mutation frequency varies by ethnicity. The I2020T is found in Japanese,(55) the R1441C, Y1699C in Caucasians(56) and the most frequently reported LRRK2 mutation, G2019S, is ubiquitous, and may be found in up to 1% of sporadic and 4% of familial PD cases worldwide.(53) Furthermore, up to 39% of Northern African Arab(57) and 18.3% of Ashkenazi Jewish(13)(58) PD cases carry the mutation. The Gly2385Arg variant, for which frequency in Chinese controls is roughly 4%, has also been associated with PD.(59)(60)(61)
Disease course may be indistinguishable from idiopathic PD. Many studies have tried to characterize mutation carriers. A single study of a North American EOPD sample reported that LRRK2 carriers were less likely to suffer from tremor and were more likely to manifest the postural instability gait difficulty motor phenotype (PIGD).(62) An Israeli study comparing presenting symptoms in G2019S mutation carriers to those in GBA carriers further reported that LRRK2 carriers were more likely to present initially with gait impairment.(63)
PARK 9: ATP13A2
Mutations in ATP13A2 are linked to a distinct syndrome, Kufor Rakeb syndrome.(64) Affected individuals develop a progressive neurodegenerative disease in their first decade including dementia, dystonia, spasticity and parkinsonism. Levodopa may improve symptoms only transiently.(65) MRI findings suggest iron accumulation in the basal ganglia; however, autopsy data are not available.(66)
Glucocerebrosidase (GBA)
While only recently identified, GBA mutations may be the most frequent genetic risk factor for PD in selected populations.(23) The association between GBA mutations and PD was initially reported in carriers of two GBA mutation, who by definition suffer from Gaucher disease.(67) More recent case-control studies indicate that even a single GBA mutation is a risk factor for PD.(68) Furthermore, GBA mutations have been linked with earlier age-at-onset.(68)(69)(70) GBA mutations are ubiquitous, but more common in Ashkenazi Jews, among whom one out of twelve carries a mutation in GBA. GBA-associated EOPD may be indistinguishable from EOPD but mutations may be associated with higher risk of cognitive impairment. In a sample of 699 North American EOPD cases, including 37 GBA carriers, mutation status was linked with self-report of cognitive impairment but not with worse performance on a cognitive screening test.(71) These findings concur with autopsy findings of cortical Lewy bodies – rather than brain-stem Lewy bodies – in GBA mutation carriers.(72)(73)
EOPD patients are less likely to have cognitive impairment.
Of note, the risk of PD in GBA mutation carriers is unknown. Furthermore, whether carrying two mutations (i.e., Gaucher disease patients) conveys a higher risk than a single mutation (i.e., heterozygotes) is also unknown.
Summary
The cause of EOPD in most individuals who develop the disease remains unknown. It is estimated that an interaction between different genes and environmental factors contributes to the development of EOPD; however, data are lacking. The overall genetic contribution to EOPD is also unknown. Very few studies have analyzed the contribution of known genetic mutations to EOPD in samples which were not ascertained by family history. An Australian study of 74 EOPD participants found a mutation frequency of 7%(74) and a Dutch study of 187 EOPD participants reported mutation frequency of 4%.(34) More recently, a study of 953 EOPD participants from 13 movement disorders centers in the United States reported a mutation frequency of 16%.(23) This research reported a higher mutation frequency in Ashkenazi Jews compared to non-Jews (32% versus 14%), in those with age-at-onset 30 or lower when compared to age-at-onset above 30 (41% versus 15%), and in those with first degree family history of PD (24% versus 14%).(23) Genotyping common GBA mutations and including participants of diverse ethnicities including Ashkenazi Jews and Hispanics, in whom mutation frequency is higher (LRRK2 G2019S in Ashkenazi Jews and PRKN in Hispanics), explain the higher frequency of mutation carriers. While much has been discovered in the past decade, most risk factors for EOPD – genetic and environmental - remain unknown.
Clinical Course of Patients with EOPD
Evaluation
The presentation of EOPD can be very variable. When it presents with rest tremor – in roughly 40% of cases(75)– diagnosis may be easier to make. However, EOPD frequently appears with dystonia or stiffness (rigidity)(75) and can be misdiagnosed. The differential diagnosis of EOPD is broad.(3) Important factors in establishing a differential diagnosis include age-at-onset of symptoms and the presence of neurological manifestations in addition to parkinsonism. If onset of symptoms is before age 30, the differential diagnosis of EOPD includes primarily metabolic and genetic disorders.(3)

Figure 1. Suggested Work-up For A Patient With Parkinsonism Below Age 30.
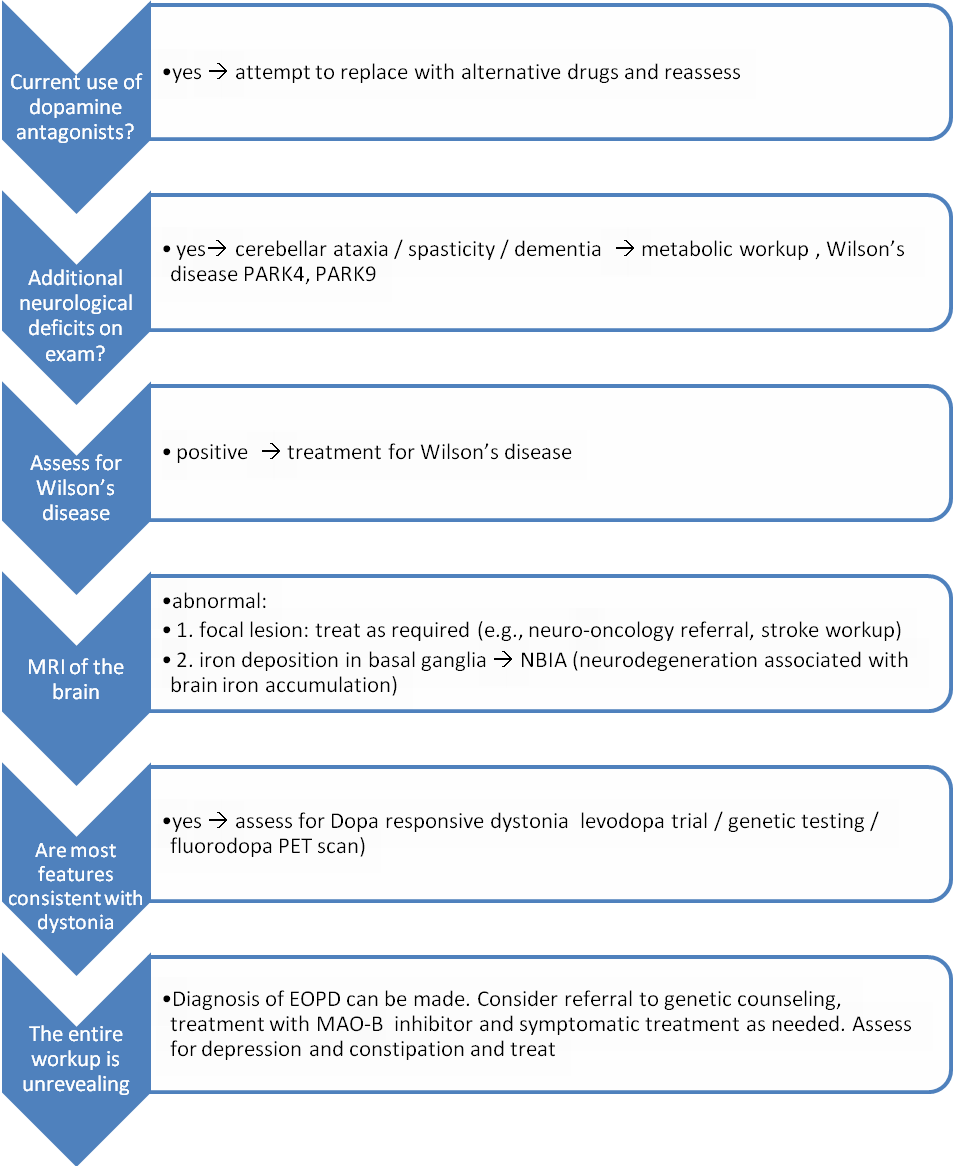
Click image for larger view.

If disease onset is 30 or older, the differential diagnosis should also include Parkinson’s Plus syndromes such as parkinsonism-dominant multiple system atrophy (MSA-P), which very rarely occurs before age 30.(76) In the evaluation of a patient with early onset parkinsonism, there are four conditions of significance that should be considered:
|
Thorough history, including family history (to rule out juvenile Huntington’s, spinocerebellar ataxia type II, spinocerebellar ataxia type III, and the above genetic forms of EOPD) and medication history, is required. If the patient is taking a dopamine blocker, either an antipsychotic medication (e.g., risperidone) or an antiemetic (e.g., metoclopramide), an attempt to replace these medications with medications with lower dopaminergic affinity (e.g., quetiapine or clozapine for delusional disorders) should be made.
The diagnosis of Wilson’s disease should also be considered, since the single most common neurological complication of Wilson’s disease is parkinsonism(77) and its treatment can significantly improve disease course.(77) Early dysarthria and personality changes may serve as clues for the diagnosis(77) but an ophthalmologic exam, including a slit lamp exam to look for a Kayser Fleischer ring as well as measurement of serum copper and ceruloplasmin levels, should be sought when the diagnosis is considered.
Diurnal variation, juvenile onset of symptoms and family history (autosomal dominant with incomplete penetrance) may suggest DRD. Often diagnosis is made through a therapeutic trial with levodopa (starting dose of 100 mg three times a day) and clinical follow-up for improvement. DRD patients will report significant improvement with treatment. DRD is not a neurodegenerative disorder and the disease may not progress over time.
Delaying levodopa treatment in EOPD seems reasonable.
The work-up for DRD can include genetic testing of the GTP cyclohydrolase gene; however, results may be inconclusive because DRD syndrome may be caused by mutations in other genes in the tetrahydrobiopterin synthesis pathway. A phenylalanine loading test is no longer performed because of lack of sensitivity. A fluorodopa PET scan may be helpful, as fluorodopa uptake is normal in DRD and reduced in EOPD. It is important to consider DRD in the differential diagnosis because its prognosis is better than EOPD’s and only a low dose of levodopa (indefinitely) may be required.
While functional imagining – including fluorodopa PET scan, dopamine active transporter (DAT) scan and single photon emission computed tomography (SPECT) – are abnormal in EOPD, anatomical imaging are usually normal in EOPD. Therefore, MRI imaging may help rule out alternative diagnoses including focal lesions, which may cause hemidystonia, and diseases of neurodegeneration associated with brain iron accumulation (NBIA).(78)

Figure 2. Suggested Work-up For A Patient With Parkinsonism Above Age 30.
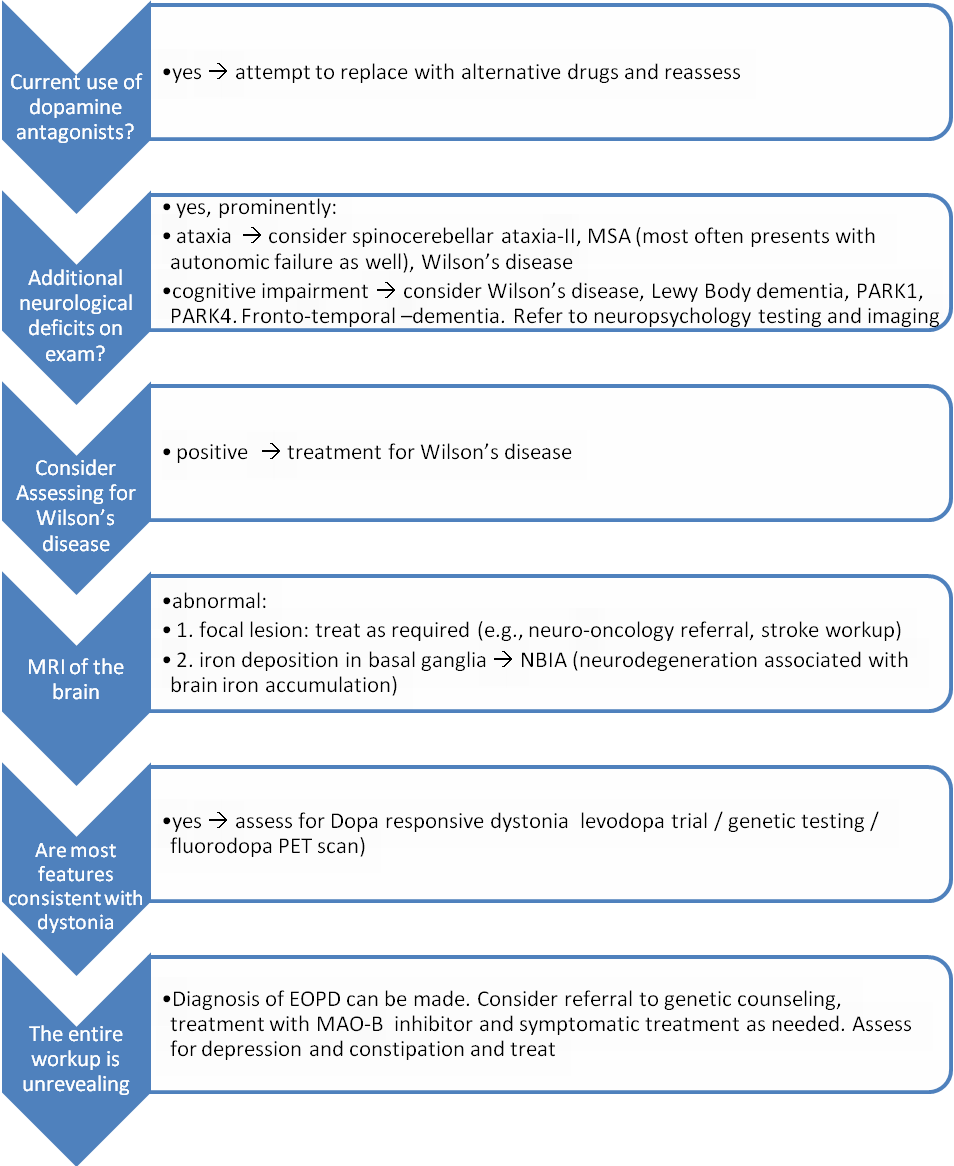
Click image for larger view.

In summary, EOPD diagnosis is based on careful history and examination. Brain imaging to rule out focal lesions when symptoms are exclusively unilateral and a specific testing for Wilson’s disease are often helpful. Long-term follow-up and medication response will further help rule out alternative diagnoses including DRD and MSA-P.
Clinical Course
The clinical features of EOPD are similar to late-onset PD. The cardinal motor features of rigidity, rest tremor, bradykinesia and postural instability are the same. However, patients may present with dystonia at onset,(25)(79) which makes the diagnosis of EOPD difficult to distinguish from dystonia in general and from dopa-responsive dystonia specifically. There are no longitudinal prospective studies comparing EOPD to late-onset PD, and most available retrospective studies do not stratify their findings by the genetic status of the participants.
In general, disease progression is slower(80)(81) and patients with EOPD may be more likely to suffer from dyskinesias as a complication of dopaminergic therapy than patients with late-onset PD;(82) however, the association between EOPD and the development of dyskinesia was inconsistently reported(75) and may be related to the longer disease duration expected in patients with EOPD.
In addition to milder motor progression, EOPD patients are less likely to have cognitive impairment.(83) Dementia is rare in EOPD, at least in its early stages when studied in a community-based sample.(84) It is unknown whether EOPD patients are less likely to dement than individuals with late-onset PD or if the frequency of dementia among EOPD cohorts was low because of their younger age at the examination. In spite of the milder motor and cognitive profile of EOPD, psychiatric co-morbidities are common in EOPD and most often include depression and anxiety.(83)
The impact of Parkinson on EOPD individuals differs from its impact on late-onset PD individuals.(85) Many EOPD individuals are first diagnosed when their young children are still living at home and require their care. Not infrequently, they are forced to retire early.(3)(85) Motor impairment from the disease, therefore, impairs their lifestyle in a way which is more disruptive than in late-onset PD.(85) Mortality in EOPD is estimated to be at least two times that of the unaffected population,(83) and disease duration is therefore highly variable. It has been described to range between 10-40 years;(83)
Treatment
Symptomatic treatment of EOPD is similar to that of PD, though in some cases (e.g., PRKN) the dose of levodopa required for symptomatic treatment is low, and because they are young, higher doses of levodopa can be given as needed.(86) Currently, there is no FDA-approved disease modifying therapy. Therefore, treatment should be tailored per signs and symptoms with a primary goal of keeping patients independent as long as possible. The initial decision in the management of EOPD is whether pharmacological treatment is warranted.(86) Levodopa in combination with an inhibitor of L-aromatic amino acid decarboxylase (DDI) is the most effective treatment for PD.(87) However, many clinicians would not introduce it as the first line of treatment in EOPD for several reasons:
|
Patients with EOPD are expected to live up to four decades with the disease.
Given that EOPD individuals are expected to live for 10-40 years with the disease, delaying levodopa treatment in EOPD seems reasonable. Alternative treatments include anticholinergics, monoamine oxidase B inhibitors (MAO-B inhibitors), amantadine and dopamine agonists. MAO-B inhibitors, including selegiline and rasagiline, are of special interest. Some studies suggest that selegiline and rasagiline may have a disease modifying effect.(90)(91)
Describing the treatment options for PD is beyond the scope of this Cyberounds®; however, a proposed treatment plan would include postponing pharmacotherapy as long as symptoms are mild. When needed, treatment with MAO-B can be initiated. In addition, either amantadine, or a dopamine agonist (both ropinorole and pramipexole are approved in the United States) can be added. Side effects of dopamine agonists, including impulse control disorders, daytime somnolence and edema should be monitored.(92) When symptoms can no longer be controlled by dopamine agonists or if side effects develop, levodopa in combination with DDI should be introduced. Lastly, many patients with EOPD will develop motor side effects,(82) including severe fluctuations in motor function and dyskinesia, and may benefit from deep brain stimulation (DBS).(86)
Non-motor complications of EOPD should be assessed and treated, including constipation, orthostatic hypotension and cognitive impairment. Of all non-motor complications, depression can be very debilitating and can potentially be treated. Given the high frequency of depression in EOPD,(3) careful screening and prompt treatment are recommended. Phase II data support treatment with nortriptyline(93) and serotonin specific reuptake inhibitors (SSRI) are also often used.
In sum, patients with EOPD are expected to live up to four decades with the disease. Careful treatment with dopaminergic and non-dopaminergic treatment may improve their quality of life and prolong their life expectancy.(86)
Summary
Parkinson’s disease is a degenerative disorder. Its presentation at early age of onset presents a challenge both in differential diagnosis and in management. Affected individuals are expected to live with motor impairment for 10-40 years. In addition, the risk of cognitive impairment and depression may further impact quality of living. As in early-onset Alzheimer’s disease, clues to genetic causes of the disease may be forthcoming from these individuals.