Course Authors
Robert J. Pignolo, M.D., Ph.D.
Dr. Pignolo is Assistant Professor and Director, Ralston-Penn Clinic for Osteoporosis & Related Bone Disorders, Department of Medicine, Division of Geriatric Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA.
Within the past 12 months, Dr. Pignolo reports no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss how rates of senescence and primary aging processes influence life span
Define the differences between average life span, median length of life, maximum life span and life expectancy
Evaluate the key factors associated with exceptional human longevity.
Aging refers to any time-related process that occurs during the life of an organism including those that promote beneficial, neutral and deteriorative consequences. Although often used interchangeably with the term aging, senescence specifically refers to those deteriorative changes that occur with time during postmaturational life that increase vulnerability to disease and decrease the likelihood of survival.
Rates of Senescence and Primary Aging Processes
There is no strong evidence that prokaryotes (e.g., bacteria) undergo senescence. Populations of single-celled eukaryotic organisms, such as budding yeast, are also immortal, but individual cells within these populations have a limited life span as measured by the number of bud generations or cell divisions. In multicellular organisms, senescence is thought to occur in those species where the germ cell line is separate from the somatic (body) lines, in those displaying a distinction between parent and smaller offspring or, more precisely, in those whose cells undergo somatic cell differentiation (by which cells take on a specialized function during growth and development).
Senescence can be described among different species as rapid, gradual or negligible. Rapid senescence occurs suddenly with deteriorative changes soon after maturation in nematodes, flies and in other short-lived invertebrates, or soon after reproduction in species such as annual plants and Pacific salmon. Negligible senescence is exhibited by long-lived species such as clams, trees, fish and reptiles, in which there is little evidence for postmaturational increases in mortality rates. Gradual senescence progresses persistently but slowly after maturation in all placental mammals including humans. However, measurements necessary to corroborate the occurrence of senescence are complicated and are often confounded by predation, infection, other environmental factors that predispose individuals to very high accidental death rates and relatively long life spans in some species. Despite these limitations, senescence is thought to occur largely as the result of primary aging processes, that is, aging in the relative absence of disease or injury.(1)
Measuring Life Span
Mean or average life span is distinguished from median length of life, which is the age at which there are as many individuals with shorter life spans as there are individuals with longer life spans. Life expectancy is the expectation of a certain mean length of life at birth, or at any age, calculated from the current mortality conditions in a population. For example, the life expectancy for a hypothetical group of 70-year-olds would be based on the currently observed age-specific death rates among individuals age 70, 71 and so on, up to the greatest age attained in the prevailing population. Maximum life span is the age of the longest-lived survivors of a cohort or population; for humans, it is operationally considered to be the oldest age reached by one in 100 million people.
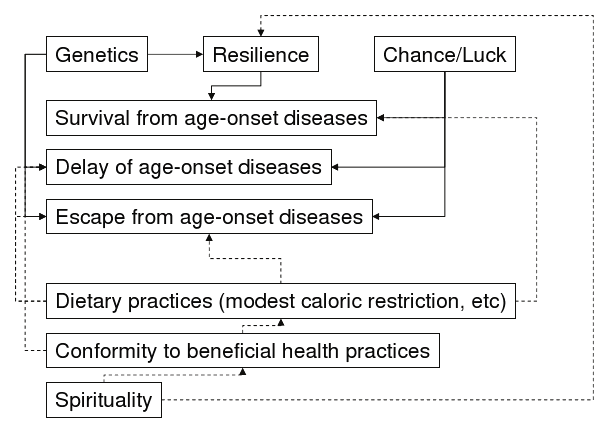
Protection from premature death, rather than changes in aging processes, underlies the survival increases reflected by median life span and life expectancy (Figure 1). Reduction in the high rate of infant deaths by improvements in sanitation, nutrition and immunization account for much of the protection from premature death by environmental hazards and infectious diseases seen early in the last century.(1)

Figure 1. Increase in Average Life Span.
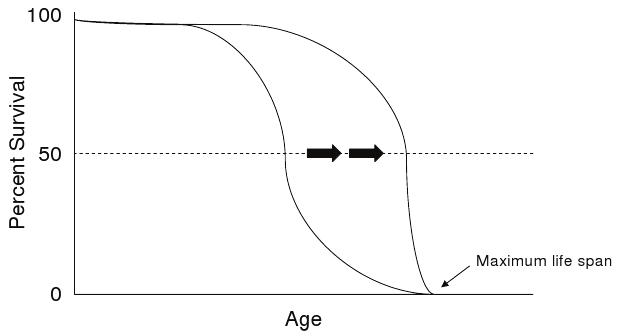
Click image for larger view.
Increase in average life span (black arrows), but not maximum life span, is the result of protection from premature death.

Median life span and life expectancy are thus influenced by many factors and are thought not to reflect primary aging processes. However, in 2008, life expectancy at birth exceeded 80 years in 11 countries, with twentieth century life expectancy doubling in some developed countries.(2) Although rising life expectancy at birth is not universal, the current highest recorded average life expectancy is for Japan where it continues to rise.(3) In fact, female life expectancy in Japan has risen for the last 160 years at a steady pace of almost 3 m/yr. Figure 2 illustrates the average life expectancy in selected developed countries.

Figure 2. Life Expectancy At Birth (2008).
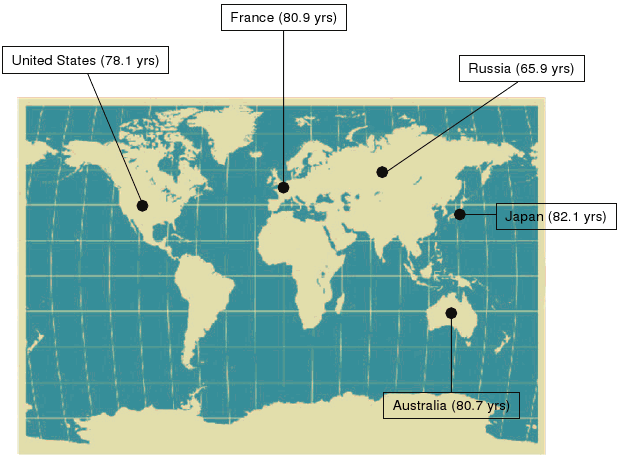
Click image for larger view.
Life expectancy at birth in 2008 in five developed countries. Source: U.S. Census Bureau, International Database, accessed on May 27, 2008.

Despite some challenges to the contrary, maximum life span has been considered an index of the rate of aging of a population and the maximum life span of a species tends to be inversely related to its rate of aging. For example, rats, with a maximum life span of about five years, are thought to age more rapidly than dogs, with a maximum life span of about 20 years. Any factor that increases the maximum life span of a species is considered to have influenced primary aging processes.
Centenarians
The number of centenarians doubled each decade between 1950 and 1990.(4) Vaupel and Jeune estimated that over the course of human history the likelihood of living from birth to age 100 has risen from 1 in 20 million to 1 in 50 for females in low-mortality nations such as Japan and Sweden.(4) About one in every 10,000 persons in the United States is 100 years of age or older, and it is likely that most industrialized countries will see a dramatic increase in this prevalence.
Maximum human life span is greater than 115 years, with reliably reported ages of 121 years for Jeanne Calment who died in France in 1996(5) and 112 years for Antonio Todde who died in Sardinia (Italy) in 2002, two weeks before his 113th birthday.(6) Although the median length of life and life expectancy for humans have markedly increased over the last 100 years, there has been little, if any, change in maximum life span. In fact, over long periods of history maximum human life span has been relatively constant.
Factors That Contribute To Exceptional Longevity
Human subpopulations that display exceptionally long life spans illustrate the profound effects of pro-longevity factors. Long-lived Okinawans ascribe to the dietary mantra of “hara haci bu” or “eat until you are only 80% full.” Their so-called “rainbow diet” consists of a vast array of fruits and vegetables with the largest protein component composed of soy.(7)(8) Their daily caloric intake is substantially reduced compared to other diets, accounting for their low BMI (~20) and suggesting that an effect of modest caloric restriction may be at least partially responsible for their long lives. In an analysis of U.S. centenarians born in the 1880s, lean body mass was also associated with extreme longevity.(9) Levels of dehydroepiandrosterone (DHEA), the endogenous hormone secreted by the adrenal gland and a surrogate marker of life span extension, decline more slowly in Okinawans and mimic the trend seen in animal experiments of controlled caloric restriction.(10)
Compared to men elsewhere, men in Sardinia tend to live longer.(11) Sardinians who emigrated at 20, 30 or 40 years of age are still capable of extreme longevity. They appear to be descended from only a few original settlers, remained isolated and practiced interbreeding.(12) The origin of this male exceptional longevity may be related to as yet undefined genetic traits.(13)
Seventh Day Adventists, many of whom live in Loma Linda, California, live 5-10 years longer than fellow citizens.(14)(15) Religious practices include no drinking or smoking, and many adhere to a vegetarian diet that their church advises. Spiritual life remains the centerpiece of their daily living; in fact, regular churchgoers of whatever faith live longer compared to non-churchgoers.(16)(17) Seventh Day Adventists have significantly lower levels of measured stress hormones.(18)
Biomarkers, Characteristics of Aging and Heterogeneity in Long-lived Humans
True biomarkers of aging, if identified, would help us determine the biological age of individuals and estimate their life expectancies. They would facilitate monitoring the impact of various interventions on the rate of aging and standardizing studies in gerontologic research. Minimal criteria for a biomarker of aging would include (1) existence of a quantitative correlation between the biomarker and the age of subjects; (2) evidence suggesting the parameter is not altered with a disease process; (3) confidence that an age-related alteration in the parameter is not secondary to metabolic or nutritional changes; and (4) demonstration that factors which influence the aging rate also alter the putative biomarker.(19)
Organisms exhibit a spectrum of morphological and physiological changes with age, some of which may be deleterious and cause functional decline. These changes may be due to aging processes, disease processes, toxic exposures, compensatory responses to injury or physiological deficits, or some combination of these influences. A challenge of basic gerontological research is to determine which of the many characteristics of aged individuals directly or indirectly relate to aging per se and constitute primary aging processes.
Some characteristics proposed as being common to human aging include the increased mortality after maturation (e.g., survival curves showing exponential increase in mortality with age), changes in the biochemical composition of tissues (e.g., increases in lipofuscin or age pigment, increased cross-linking in extracellular matrix molecules such as collagen), progressive, deteriorative physiologic changes (e.g., declines in glomerular filtration rate, maximal heart rate, vital capacity), decreased ability to adaptively respond to environmental changes (e.g., decreased "first-pass" hepatic metabolism, blunted maximal cardiac responses to exercise) and increasing incidence of many diseases (e.g., ischemic heart disease, type II diabetes, osteoporosis, Alzheimer's disease).(20)
These "characteristics" of aging, although widely found, have exceptions which call into question their relevance to primary aging processes and their use as biomarkers for aging.(20) For example, human age-specific mortality rates in many cases do not continue to increase exponentially at very advanced ages. Alterations in biochemical composition of tissues, deteriorative physiologic changes and maladaptive responses to environmental challenges are quite heterogeneous from organ to organ within a specific individual and also from individual to individual. Although mortality rates for many diseases increase with age and parallel the exponential increase in mortality with age, at least one estimate predicts that elimination of atherosclerosis and cancer as causes of death would only add about ten years to average life span and would not affect maximum life span potential.
In centenarians, the age of onset of common age-associated diseases (excluding cognitive impairment) were found to be variable, with 24% of males and 43% of females attaining at least one diagnosis prior to the age of 80. About 43% of both male and female centenarians delayed the onset of age-associated illness until at least 80 years of age. Approximately 30% of male centenarians and half that number of female centenarians escaped any diagnosis by age 100. These results suggest that the onset of age-related conditions, regardless of sex differences, is heterogeneous and earlier onset of these conditions (prior to the age of 80) does not preclude the achievement of exceptional longevity.(21)
Disability and Morbidity With Increased Life Expectancy and Exceptional Old Age
As many as 25% of centenarians are cognitively intact, and among those who reach 100 years of age and are not cognitively intact, the vast majority of them nevertheless delay the clinical onset of impairment until the average age of 92.(22)There are centenarians who demonstrate no evidence of neurodegenerative disease as well as those who, despite the presence of neuropathological markers of Alzheimer’s disease, do not meet the criteria for dementia. The age of cancer diagnosis is also substantially delayed in centenarians.(23) Supercentenarians, defined as those who survive to be over 110 years of age, delay and even escape clinical expression of vascular disease and many are still functionally independent or require minimal assistance.(24)
Fries’ “compression of morbidity” hypothesis puts forth the possibility that there would be a delay in the onset of chronic morbidity and that the delay of this onset would be greater than the increase in life expectancy.(25) In other words, if individuals live longer, they would have a reduced cumulative morbidity by compressing the number of years they would suffer from chronic diseases and associated disability.
Evidence that a decline in disability trends of about 2% per year, accompanied by a 1% per year decline in mortality during the same period of time, supports this hypothesis.(26) With the fastest growing segment of the American population being 85 years and older, there is concern that compression of morbidity and disability might not hold true for those who survive to exceptional old age. Delays in morbidity from age-onset diseases occur in many of those who survive to very old age; for others who survive to extreme old age, delays in disabilities alone are the important requirement.(27)
The Genetic and Evolutionary Basis of Longevity
The genetic basis of longevity is supported by the high conservation of maximum life span seen between species, by the similarity of attained age between monozygotic twins compared to dizygotic twins or non-twin siblings, by examples of exceptional longevity within families, and by the presence of subsets of aging features in human genetic diseases of premature aging. Twin studies demonstrate that genetic differences likely account for about 25% of the variance in adult human life span.(28) Centenarian offspring have an increased likelihood of surviving to 100 years and like their parents show a reduced prevalence of age-associated diseases.(29) Compared to reference individuals who lived at the same time and in the same place as the ancestors of Jeanne Calment, there existed an extraordinary group of long-lived relatives within her family’s last five generations, especially in her father’s lineage, suggesting a largely genetic origin for her extreme longevity from paternal inheritance.(5)
Common polymorphisms that have a modest effect on life span have been identified in the APOE gene, essential for the normal catabolism of triglyceride-rich lipoprotein constituents. There is a higher frequency of the apolipoprotein E epsilon4 allele in middle-aged subjects compared to centenarians. Polymorphisms in other genes and their associated pathways implicated as imparting pro-longevity effects include insulin/insulin-like growth factor-1 (IGF-1), cholesteryl ester transfer protein (CETP), anti-inflammatory cytokines such as IL-10, RNA editing genes and stress response genes such as the heat shock protein (HSP)-70 genes.(30)(31)(32)(33)(34)(35)(36) Polymorphisms in genes protective against oxidative stress such as superoxide dismutase have not been found to be associated with long life. The GenAge database (http://genomics.senescence.info/genes/) gives a comprehensive listing of genes analyzed for their possible association with human longevity (http://genomics.senescence.info/genes/longevity.html).
In species where the germ line is separate from somatic tissue, the risk of mortality increases with time after reproduction. This may be based on selective pressure to retain genes with a positive effect on reproductive fitness in young individuals. Thus genes that confer early beneficial effects on reproduction would be selected by evolution, even if they account for deleterious effects in later life. Conversely, there is no selective pressure against genes that confer negative effects in later life, subsequent to reproduction and care of the young after birth. However, there would be strong selective pressure to retain genes that diminish vulnerability in the young and old alike, which would both increase reproductive success and produce increases in longevity.
Evidence in favor of evolutionary theory comes from studies in flies and lower mammals, where either intentionally or by examples of reduced predation in nature, respectively, the decreased need for early, rapid and prolific reproduction caused extended life spans. Similarly, the exceptional longevity of birds and turtles, relative to comparably sized mammals, is thought to be related to the selection of genes which favor later reproduction, independent of predator-free environments.
Evolutionary pressure to extend human life span may also be closely linked to prolonging the period of time during which women can bear children.(37) The length of a female’s post-reproductive life span also appears to be reflected in the reproductive success of her offspring and the survival of her grandchildren, although this so-called “grandmother hypothesis” may be more relevant in the case of maternal grandmothers.(38)(39) In a study of centenaries who lived in the U.S in the 1980s, large numbers of children, but not martial status, was associated with long life.(9)
Interventions in the Aging Process: Implications for Life Span Extension
The most convincing dietary therapy to retard most aspects of aging in mammals is caloric restriction.(40) A 30 to 60 percent reduction of calories can produces extensions of both mean and maximum life span (Figure 3).

Figure 3. Extension of Average and Maximum Life Spans.
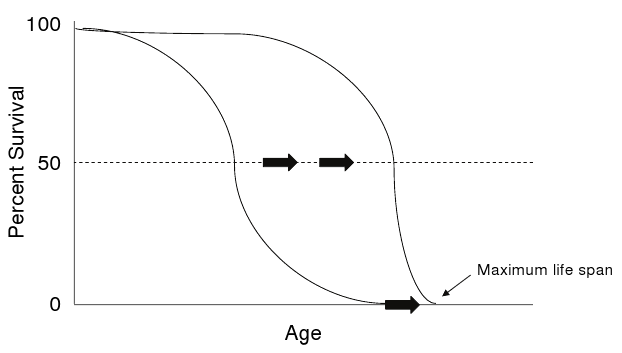
Click image for larger view.
Extension of average and maximum life spans (black arrows) [e.g., by interventions such as caloric restriction] is thought to reflect changes in primary aging processes.

This effect has been reproduced in controlled studies in a variety of species including worms, flies, fish, rodents and most recently in non-human primates. Findings of a 20-year longitudinal adult-onset caloric restriction study in rhesus monkeys showed that moderate reduction in dietary intake lowered the incidence of aging-related deaths and delayed the onset of age-associated pathologies including diabetes, cancer, cardiovascular disease and brain atrophy.(41) Short-duration studies of caloric restriction in humans report beneficial outcomes in surrogate markers of delayed aging such as improvements in serum glucose and insulin levels.(42)(43)
The degree of caloric restriction needed to achieve a true anti-aging effect is probably too severe to be viewed as a practical regimen in all but a very small fraction of the population. Therefore, it will become necessary to define the mechanism(s) by which caloric restriction alters age-dependent processes that cause functional decline. Two other dietary manipulations also extend maximum life span, low-methionine diets and brief, but early, nutritional deprivation.(44)(45) In the case of a low-methionine diet, its pro-longevity effects cannot be accounted for by diminished caloric intake. Dietary supplementation with antioxidants has not yet been shown to significantly change median or maximum life span.
The National Institute of Aging has organized a multi-site study of pharmaceuticals that have the potential to extend life span in genetically heterogenous mice. Of the agents being tested, aspirin and nordihydroguaiaretic acid have been found to lead to significant increases in life span in males,(46) while rapamycin leads to an increase in maximum longevity in both males and females.(47) Other compounds currently being tested as part of this initiative can be found at http://www.nia.nih.gov/ResearchInformation/ScientificResources/CompoundsInTesting.htm.
Successful genetic manipulations that may influence life span have been described in both mammalian and invertebrate species.(48) The "Ames dwarf" mutation produces a developmental deficiency of the pituitary when mice receive two copies of the mutant allele. Mice homozygous for the mutation have a near-total absence of growth hormone, prolactin, and thyroid-stimulating hormone, as well as a one-third reduction in normal body weight. These mice have up to a 68 percent increase in life span, depending on sex. Another mouse mutation, the "Snell dwarf" mutant, leads to low growth hormone production, a very small body size and dramatic life span extension. Long-lived strains of fruit flies and nematodes have been obtained by selective breeding. Length of life has been increased in nematodes and yeast by specific single-gene mutations and fruit flies exhibit extended life when made to overexpress both superoxide dismutase and catalase. It is unclear how these genetic changes relate to human longevity.
Other manipulations including reduction in body temperature and voluntary exercise have also been shown to affect life span. As examples, a low body temperature increases median and maximum life span in many poikilothermic animal species (animals whose body temperatures fluctuate with the environment) and voluntary exercise in rats increases average life span. Habitual exercise does increase healthy life span in humans but whether it also extends the maximum length of life remains an open question.(49)(50)(51)
Summary
Exceptional longevity represents an extreme phenotype. Centenarians are survivors of a cohort who escaped infant mortality, infectious illnesses in the pre-antibiotic era and fatal outcomes of common age-related diseases. They exhibit marked delay of onset, escape, or otherwise survive age-associated diseases that typically cause mortality at earlier ages. The mechanisms of extreme long life appear to be multi-factorial (Figure 4), and can be accomplished by disparate combinations of genes, environment and chance that vary with culture and geography.