Course Authors
Thomas C. Arnold, M.D., and Robert A. Barish, M.D., M.B.A.
Dr. Arnold is Professor and Chairman, Department of Emergency Medicine, and Dr. Barish is Chancellor, the LSU Health Sciences Center, Shreveport, LA.
Within the past 12 months, Dr. Arnold and Dr. Barish report no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
List the presenting signs and symptoms of snake bites in North America
Discuss important aspects in pre-hospital and hospital management of the snakebite victim
Describe the risks and benefits of antivenom therapy after snakebite and its indications
Discuss the challenges associated with currently available antivenom products and future directions in antivenom development.
Worldwide, an estimated five million people are bitten by snakes every year, and up to 125,000 envenomations result in death.(1)(2) In contrast, in the United States, 7,000 to 8,000 people are bitten by venomous snakes each year, and five to six of those bites lead to death.(3) Throughout the world, most bites are associated with farming and food production; most bites in the United States are the result of deliberate exposure to a snake, whether captive or in the wild.(4)
Snakebites evoke a primal and innate human emotion in the victim and in many treating physicians as well.(5) With recent advances in antivenom production techniques and the worldwide recognition of snakebite as a neglected tropical disease,(6) it has become important for physicians to have a working knowledge of the epidemiology, pathophysiology and emergent medical management of this disease.
Identification
Only poisonous snakes from the Crotalinae subfamily (pit vipers) and the coral snake from the Elapidae family are native to North America. But countless varieties of non−native snakes are brought into the United States by collectors and zoos―both legitimate and not.(7) Many of these collections contain exotic snakes such as cobras and vipers, for which antivenoms may be difficult or impossible to obtain.
The North American pit vipers include species of the Crotalus and Sistrurus genera (rattlesnakes) and two from the Agkistrodon genus (cottonmouth, copperhead). All pit vipers possess hollow fangs; slit−like, elliptical pupils; and a heat−seeking “pit” between each eye and nostril. Since the head of the snake presented when the victim seeks treatment is often not identifiable, it is important for practicing physicians to be familiar with the color patterns of snakes in their region. The pit vipers have a different scale pattern distal to the anal plate than non−poisonous varieties. The anal plate is located roughly three fourths down the body on the underside of the snake. Pit vipers have a single row of scales beyond this landmark before turning to a double−row near the very end. Nonpoisonous varieties begin this “double row” of scales immediately after the anal plate. Identification assistance can be obtained from the local Poison Center at 1−800−222−1222 or a local zoo herpetologist.
In contrast to the pit viper, the coral snake has no heat−seeking pit, grooved teeth instead of hollow fangs and round pupils. The three North American varieties are easily distinguished from the non−poisonous scarlet king snake by the color pattern mnemonic “red on yellow, kill a fellow; red on black, venom lack.”
Pathophysiology
Snake venoms are a complex mixture of enzymatically active proteins that vary in size and target receptor sites.(8) Predominant effects induced by the North American pit vipers include damage to endothelial cells of vascular and lymphatic structures, and hematologic destruction of blood components. The quantity and potency of the venom vary among species. Even within species, venom makeup varies by geographic location, age of the snake, time of year and many other factors, including genetics.(9) Venom itself is very stable over time to temperature extremes, barometric changes and dessication.(10)(11)
Almost every organ system is affected by some venom component. Although predominant effects may be categorized as hemotoxic, neurotoxic, cardiotoxic or myotoxic,(12) it would be misleading to give any particular venom one of these labels, since there is a wide overlap of effects across all species. For example, Mojave rattlesnake venom produces few local tissue effects and some delayed neurotoxic symptoms. In contrast, the coral snake's venom has only neurotoxic effects.
The venom of pit vipers increases capillary membrane permeability, resulting in extravasation of red cells, proteins and electrolytes into the tissues around the bite and later in distant organs and tissues, such as the lungs, heart and kidneys, as well as the central nervous system. Altered red cell permeability leads to hemolysis and a cascade of microvascular insults, resulting in hypovolemic shock and lactic acidosis. Renal failure and disseminated intravascular coagulation are often the result of the hypotension and hemolysis.
Clinical Presentation
Pain from envenomation by a pit viper is almost immediate and is often described as an intense stinging sensation or blow from a hammer. Up to 25% of pit viper bites are “dry” (no appreciable venom is injected into the victim). Venom effects range from minimal to severe.(13) These effects can remain local, become systemic and/or manifest as coagulation abnormalities (see Table 1).

Table 1. Guidelines for Assessing the Severity of North American Pit Viper Envenomations.
| Type of Signs or Symptoms | Severity of Envenomation |
||
|---|---|---|---|
| Minimal | Moderate | Severe | |
| Local | Swelling, erythema, and/or ecchymosis confined to the bite site. | Evidence of progression of swelling, erythema, and/or ecchymosis beyond the bite site. | Rapid swelling, erythema, and/or ecchymosis involving the entire extremity |
| Systemic | No systemic signs or symptoms | Non-life-threatening signs/symptoms, (nausea, vomiting, perioral paresthesia, myokymia, mild hypotension) | Markedly severe signs/symptoms (hypotension [SBP <80 mm Hg], altered sensorium, tachycardia, tachypnea, or respiratory distress) |
| Coagulation | No coagulation abnormalities or other important laboratory abnormalities | Mildly abnormal coagulation profile without clinically significant bleeding; mild abnormalities in other laboratory tests | Markedly abnormal coagulation profile with evidence of bleeding or threat of spontaneous hemorrhage (unmeasurable INR or APTT, and fibrinogen; severe thrombocytopenia with platelet count <20,000 per mm3); results of other laboratory tests may be severely abnormal |
The ultimate grade of severity of any envenomation is determined on the basis of the most severe sign, symptom, or laboratory abnormality (e.g., systolic blood pressure <70 mm Hg in the absence of local swelling should be graded as a severe envenomation).
SBP, systolic blood pressure; INR, international normalized ratio; APTT, activated partial thromoboplastin time.
From Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med 2002; 347(5):347-356. Copyright © 2002 Massachusetts Medical Society. All rights reserved.

Victims of pit viper envenomations commonly present with one or more fang marks as puncture wounds or scratches. Continued oozing of blood from the fang marks is supporting evidence of an envenomation, as the hemotoxic effects of the venom begin manifesting locally. Venom spreads from the bite site via the lymphatic system. The leading edge of this lymphatic spread can be readily observed, measured, marked and timed as an indicator of bite severity. Ecchymosis and hemorrhagic bullae are commonly seen within several hours after the bite, along with signs of lymphangitis and tender regional lymph nodes. Early evidence of systemic involvement may be noted by perioral parasthesias, lethargy, hypotension, tachycardia, respiratory distress and altered sensorium. Rattlesnake bites, particularly, may result in a consumptive coagulopathy manifested by unmeasurable coagulation indices, platelet counts below 20,000/µL and hypofibrinogenemia.
Treatment
Pre−hospital
Victims of a venomous snakebite should move away from the snake immediately to avoid repeated bites. Capturing the snake for positive identification is not necessary for medical treatment purposes and frequently results in multiple victims. Efforts should be made to calm the patient while arranging transport to the nearest appropriate healthcare facility. Although no studies have measured outcomes, it makes intuitive sense to keep the immobilized affected body part in a neutral or slightly dependent position in relation to the heart.(14) Any potentially constricting clothing or jewelry should be removed immediately before edema develops. Outdated first−aid measures such as incision and suction, application of a tourniquet, immersion in ice and electric shock therapy have all been shown to be of no value and potentially dangerous, so these practices should be abandoned.(14)(15)(16)(17)(18) First responders should direct their attention to supporting the airway, breathing and circulation (ABCs), and establishing intravenous access in an unaffected extremity while transporting the patient without delay.
Emergency Department
The two main goals in treatment of snakebite victims are supporting the ABCs and timely administration of antivenom when indicated. A brief history should be obtained, focused on the specifics of the bite, the type of snake and the patient's medical conditions and allergies, specifically those to horse or sheep products. Initial evaluation should include close inspection of the bite site for evidence of fang marks, swelling and ecchymosis. The leading edge of edema should be measured and marked upon arrival and every 15 minutes to document progression as a guide for antivenom administration.(12) Blood should be drawn for baseline measurement of platelets, complete blood count, coagulation profile (PT/INR, PTT) and fibrinogen. Routine electrolytes with renal function tests and urinalysis should also be collected. Other laboratory and diagnostic studies may be indicated based on the severity of the envenomation or existence of co−morbid diseases.
Patients with a suspected pit viper bite should be observed for at least 8 hours prior to discharge, since delayed manifestations of envenomation may develop. The decision to treat with antivenom is made after careful consideration of multiple factors, including the species of snake (if known), rapidity of progression of edema from the bite site, presence of any systemic findings and laboratory coagulation abnormalities. Consultation with a toxicologist or the local Poison Center is recommended for assistance regarding antivenom dosing.
Coral snake envenomation will not induce local tissue effects, as seen with the pit vipers, and may manifest as neuropathic symptoms or respiratory compromise hours later. For this reason, asymptomatic patients with unconfirmed or suspected coral snake bites should be admitted and monitored closely, with antivenom readily available for at least 12 hours to ensure safety. Patients with confirmed bites from a coral snake should all be treated with antivenom, since the late neurotoxic effects are not easily reversed by delayed antivenom administration.(19)
Antivenoms
The only antivenom currently approved and available for treatment of North American pit viper envenomations is Crotalidae Polyvalent Immune Fab (Ovine) (FabAV) under the trade name of CroFab™. It is a sheep−derived polyvalent antivenom produced by immunizing sheep with venom from four species of pit vipers.(20) Strict indications for use of CroFab™ have not been clearly demonstrated but, as familiarity and comfort with the safety profile of the product develop, physicians are utilizing antivenom therapy earlier and more often in envenomations previously not treated because of fear of anaphylactic reactions.(21) Bites from rattlesnakes resulting in progressive local symptoms, laboratory abnormalities or evidence of systemic venom effects are generally regarded as appropriate for antivenom therapy.
Key to the antivenom dosing regimen is the concept of “initial control” followed by a scheduled course of maintenance doses. This initial control is defined as “the cessation of progression of all venom effects including local, systemic and coagulation abnormalities."(22) Doses of antivenom should be repeated consecutively in 4− to 6−vial increments until initial control is established. In most patients with rattlesnake bites, initial control is obtained with 8 to 12 vials but more may be required for severe envenomations. In patients with non−rattlesnake pit viper bites, initial control is usually obtained with fewer vials.(21) Scheduled maintenance doses of two vials each should begin 6 hours after the last dose required to attain initial control. The relatively small size of the Fab (fragment, antigen binding) molecule permits this antivenom to be cleared renally; therefore, maintenance doses are needed for 18 hours to maintain adequate tissue levels (see Figure 1).

Figure 1. Clinical Use of Crotalidae Polyvalent Immune Fab (Ovine) (FabAV).
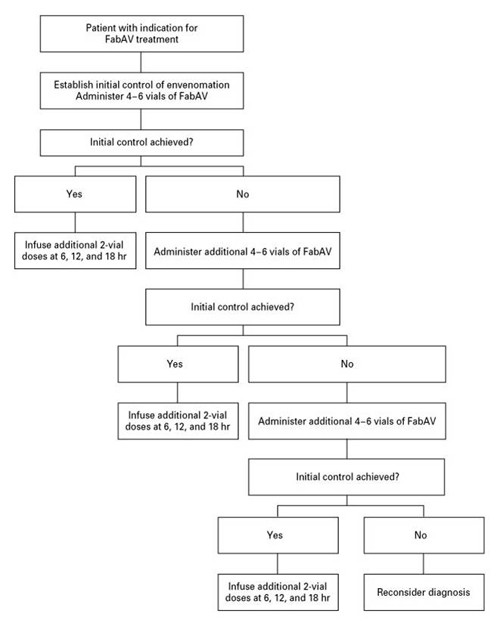
Each vial is reconstituted and the entire dose diluted to a volume of 250 ml in a crystalloid fluid for administration over the course of 1 hour. (From Gold BS, Dart RC, Barish RA. Bites of venomous snakes. N Engl J Med 2002;347[5]:347−356. Copyright © 2002 Massachusetts Medical Society. All rights reserved.)

Wyeth Antivenin (Micrurus fulvius)®, a horse−derived product, is currently the only antivenom for coral snake envenomations approved by the U.S. Food and Drug Administration (FDA). Unfortunately, Wyeth recently discontinued production and support of the product. The final production batch has been granted an expiration continuance until October 31, 2010. At the time of this writing, the FDA was wrestling with how to proceed in light of the looming catastrophe that will no doubt occur once coral snake antivenom is no longer available in the United States. An equine−based F(ab)2 product available in Mexico has been shown to be effective against the North American species but, considering the relatively small number of coral snake bites per year in the United States (roughly 100), an adequate clinical trial of this product would be logistically difficult and financially impractical.
The History and Future of Antivenom Therapy
In some of the earliest experiments in what was then termed "serotherapy," Sewell, in 1887, used repeated injections of pygmy rattlesnake venom into pigeons to demonstrate they could resist up to six lethal doses of venom. Around that time Calmette, a disciple of Louis Pasteur, developed an immunization protocol that was duplicated by many others to obtain antisera capable of inducing protection against a variety of poisonous snakes worldwide. Second−generation antivenoms contained purified whole immunoglobulin G and were represented in the United States by the Wyeth Antivenin Crotalidae Polyvalent for pit vipers and the above−mentioned discontinued coral snake antivenin also produced by Wyeth. Because of the incorporation of the antigenic Fc (fragment, crystallizable) portions on the immunoglobulins, patients given these second−generation antivenoms suffered from high rates of allergic reactions, serum sickness and occasionally true anaphylaxis. The third generation of antivenoms was ushered in by the use of papain or pepsin to separate the active Fab fragments from the antigenic Fc portions, thereby producing far fewer allergic consequences than had been previously experienced. Cleavage with papain produces single Fab fragments, while pepsin cleavage produces a F(ab)2 fragment roughly twice the size of a single Fab fragment.
Molecular size has implications on tissue penetration and renal clearance. The purification of these fragments for use as antivenoms has been termed "fabotherapy." Major breakthroughs, such as determining the full amino acid sequence of a human antibody and production of the first murine monoclonal antibody in the 1970s, quickly led to the production of transgenic mice capable of producing human antibodies. Between 1986 and 2002, the FDA approved 40 therapeutic antibodies for human use. By 2005, at least 33 transgenic antibodies were in various stages of clinical trials. A clinical trial is currently underway in the United States to compare a horse−derived product consisting of F(ab)2 fragments with CroFab™, a Fab product. It is yet to be determined whether the larger molecular size of the F(ab)2 product will affect dosing requirements or obviate the need for maintenance doses.
Recent antivenom research suggests that the Fc fragments, which were removed from the third−generation antivenoms because of their antigenic properties, may actually serve an important role in antibody–antigen interaction and immune adherence.(23) Transgenic animal models may provide the solution to the problem of allergic reactions to foreign Fc fragments. One area of investigation is focused on developing an animal model large enough to produce an adequate antibody response (e.g., a transgenic cow) so that harvesting would be commercially feasible.(24) These transgenic animals would actually produce human antibodies and, therefore, at least theoretically be free from allergic consequences. Other efforts are aimed at stimulating the host immune response toward a specific selected antigen for which the antibody is required. This process would more specifically direct the antibody production toward a more effective and desirable antivenom product.(25)
Conclusion
Although the problem of snakebite in the United States is relatively minor compared with worldwide statistics, it has persisted, so physicians should be knowledgeable as to its management and the issues related to antivenom use and development. The keys to proper treatment involve supportive care and timely use of adequate and appropriate antivenom therapy. As safer antivenoms are developed and distributed, it is conceivable that death from snake envenomation could be eliminated in this country for all patients who receive timely care.
Acknowledgment
The manuscript was copyedited by Linda J. Kesselring, MS, ELS, the technical editor/writer in the Department of Emergency Medicine at the University of Maryland School of Medicine in Baltimore.