Course Authors
Daniel Scheurich, M.D., and Keith Woeltje, M.D., Ph.D.
Dr. Scheurich is clinical fellow, Department of Medicine, and Dr. Woeltje is Associate Professor, Infectious Diseases, Washington University School of Medicine, St. Louis, MO.
Within the past 12 months, Drs. Scheurich and Woeltje report no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss a shift in etiologic agents in skin and soft tissue infections.
Enumerate risk factors for carriage and development of infections caused by CA-MRSA.
Describe surgical and pharmacologic methods of treatment of skin and skin structure infections caused by CA-MRSA.
List and evaluate methods of eradication of CA-MRSA carriage.
Staphylococcus aureus is a virulent organism that is the causative agent of a wide range of clinical disease, including skin and soft tissue infections (SSTI). Traditionally, there were two categories into which these infections fell: infections acquired in the community and those acquired in the health care setting. In this context, organisms causing community-acquired infections were typically susceptible to traditional antibiotics of choice, namely anti-staphylococcal penicillins, of which methicillin is the parent drug. These organisms are referred to as methicillin-sensitive Staphylococcus aureus (MSSA). Infections acquired in the hospital or other health care settings, in contrast, were often caused by strains of S. aureus that were frequently resistant to the beta-lactam antibiotics and were hence known as methicillin-resistant Staphylococcus aureus (MRSA).
Over the last decade there has been an increasing percentage of isolates from the community that are methicillin-resistant in patients without traditional risk factors for MRSA (such as hospitalization). The agent in these infections is now termed community-acquired MRSA (CA-MRSA). CA-MRSA infections have become epidemic in the US. Several recent series have examined the resistance patterns from S. aureus isolated from community-acquired infections and found the percentages that are methicillin-resistant range as high as 44-59%. (1)
SSTI, including simple cellulitis, are primarily caused by group A streptococcus and S. aureus.(2) Cellulitis has been a challenge to characterize etiologically because causative organisms are difficult to culture from the site of infection and peripheral blood cultures are positive in only about 2% of patients.(3) Community onset SSTI has been treated historically with oral beta-lactam antibiotics. However, with the emergence of CA-MRSA, knowledge of the pathogenesis, presentation and treatment of SSTI, including antibiotic resistance patterns, is important to a wide range of physicians. This includes primary care doctors, emergency room physicians, surgeons and infectious disease specialists, all of whom are involved in the diagnosis and treatment of these infections.
Virulence
CA-MRSA strains are particularly adept at causing SSTI, generally presenting as cutaneous abscesses. There are several important genomic distinctions between CA-MRSA strains and historical healthcare-associated strains which may lend insights into the pathogenesis of CA-MRSA.
Many CA-MRSA strains produce increased cell-surface binding proteins that may facilitate entry into non-phagocytic host cells. Pyrogenic supertoxins, encoded on the genome, may additionally help these organisms evade host immunity by preventing clonal proliferation of specific anti-staphylococcal T-cells. While these virulence factors are important in CA-MRSA pathogenicity, they do not completely explain their tendency to cause SSTI.(4)
Arginine catabolic mobile element (ACME) is a genetic component found integrated into the bacterial chromosome of many CA-MRSA isolates. It elaborates gene products that aid in the utilization of arginine. While there are also non-mobile chromosomal gene products in S. aureus performing similar tasks, they are genetically distinct. ACME is not found in hospital strains of MRSA, epidemiologic evidence for its importance in the virulence of CA-MRSA. In addition, ACME is common in Staphylococcus epidermidis, a commensal of the skin, and so its elaboration as a mobile gene product in CA-MRSA may partially explain the skin tropism of the organism. The specific role of ACME in the pathogenesis of CA-MRSA has not been determined.(4)
Panton-Valentine leukocidin (PVL), a pore-forming leukocidin, is the most common virulence factor noted in association with CA-MRSA. Like ACME, it is epidemiologically implicated in the pathogenesis of CA-MRSA, for it is present in community-acquired strains but rarely seen with healthcare-associated strains. However, PVL-knockout strains can cause abscesses in laboratory animal models, and so the importance of PVL in CA-MRSA's tendency to cause purulent SSTI is uncertain.(4)(5) Conversely, CA-MRSA strains lacking phenol-soluble modulin (PSM) peptides have reduced ability to cause abscesses in mice, implicating PSM as an important virulence factor. PSM is also produced in hospital strains of MRSA but at much lower levels in vitro. Furthermore, similar peptides are elaborated in S. epidermidis, which may again contribute to the tendency of CA-MRSA to cause SSTI. (4)
In addition to specific gene product virulence factors, regulation of gene production may be of importance in the virulence of CA-MRSA. Environmental or other factors may contribute to altered gene expression, leading to increased virulence and the organism's skin and soft tissue tropism.(4) Altered expression of gene products is another area in CA-MRSA pathogenicity which requires more study.
Risk Factors for CA-MRSA
Risk factors for colonization with and infections from CA-MRSA are poorly defined. In the initial stages of the epidemic, outbreaks were reported in military recruits, prison inmates, and participants in team and contact sports.(6) CA-MRSA is no longer seen exclusively in these groups of patients, however.
Several large studies have examined whether risk for SSTI from CA-MRSA, as opposed to MSSA, is higher in African-Americans, younger patients, those with recent B-lactam antibiotic use, intravenous drug use, homelessness and those with a recent history of incarceration. The results of these studies are conflicting -- while there are risk factors for the development of SSTI due to CA-MRSA, as opposed to MSSA, there is no reliable way to distinguish the two based on risk factors alone.(7)(8)(9)(10) CA-MRSA should be suspected in all patients presenting from the community with SSTI.
S. aureus can be a human commensal and can be recovered from the nares, axilla, vagina and groin of healthy subjects. Nasal carriage of S. aureus in healthy volunteers was found to be 23% in one study.(11) These rates may be higher in patients with exposure to health care facilities and with underlying medical illnesses, since CA-MRSA can be isolated from these same bodily areas in patients with and without a history of SSTI. Another study examined the rates of carriage of CA-MRSA in patients admitted to the hospital. Anterior nasal cultures were obtained from patients within 48 hours of hospitalization. This study found 7.3% of patients were colonized with MRSA. The study investigators were able to determine by molecular analysis that 30% of the MRSA isolates were CA-MRSA (2.2% of the total study population).(12) Analysis of data from the National Health and Nutrition Examination Survey (HANES), examining subjects who were not institutionalized, found a lower percentage of patients with nasal MRSA colonization (0.86%).(13)
Patients who develop colonization with S. aureus during hospitalization are at risk for subsequent staphylococcal infection. Approximately 10% of patients who acquired MRSA colonization in a general inpatient population developed SSTI.(13) Those patients who become prevalent carriers (carriage for greater than one year) are also at risk for subsequent infection. Twenty-three percent (23%) of such carriers developed infection in one study.(14) Of the 38% of military recruits who were found to be colonized with CA-MRSA in another study, 3% of this subgroup subsequently developed SSTI.(15)
Clinical Presentation
Infections caused by S. aureus present in a wide variety of ways, from relatively innocuous cutaneous infections to toxic shock syndrome, invasive blood stream infections, infective endocarditis, bone and joint infections, and necrotizing pneumonia. CA-MRSA tends to cause purulent skin and skin structure infections. This presentation is often somewhat different from classic cellulitis, which presents as erythematous, warm skin lesions, usually without purulence (although CA-MRSA can be a causative agent of simple cellulitis).
SSTI caused by CA-MRSA are often large, indurated or fluctuant and are located on an erythematous base. They usually spontaneously drain purulent material, although sometimes they may contain necrotic tissue with no frank purulence (Figure 1). They can be found anywhere on the body but often are seen in regions S. aureus colonizes -- on the face, in the axilla and groin regions. Lesions can be large and painful, reminiscent initially of an insect bite. In fact, patients often present with a complaint of a "spider bite", though often there is no history of a bite.

Figure 1. Partially-drained cutaneous abscess caused by CA-MRSA.
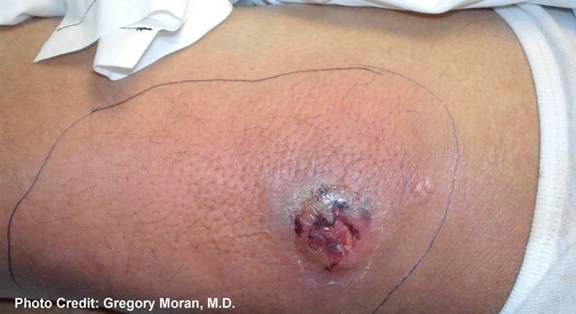
Photo courtesy of the CDC website www.cdc.gov and Dr Gregory Moran.

Recurrence is common, with a recent study showing the rate of recurrence of SSTI due to CA-MRSA at 37% versus 16% for those resulting from MSSA.(16) The reason for these recurrences is unclear. Some potential causes might involve virulence factors intrinsic to CA-MRSA, host factors including underlying immunity or personal hygiene habits or treatment decisions such as inappropriate antimicrobial use. These recurrences can make infections from CA-MRSA rather frustrating for the patient and clinician.
Like its health care-associated and methicillin-sensitive relatives, CA-MRSA can also cause deep and more invasive infections. CA-MRSA has been reported in cases of necrotizing fasciitis and infective endocarditis. CA-MRSA has also been reported as a cause of necrotizing pneumonia, an observation with clinical implications since current treatment guidelines for community-acquired pneumonia (CAP) largely ignore the possibility of MRSA as an etiologic agent.
While S. aureus historically has been estimated to cause 1-5% of CAP, this percentage may be rising in the era of CA-MRSA. Clinicians should be aware of the possibility of CA-MRSA in CAP, especially in cases involving younger patients with a previous viral-like illness, systemic inflammatory response syndrome (fever, tachycardia, tachypnea), shock, certain laboratory parameters (high or low white count, elevated c-reactive protein levels) or a chest roentgenogram showing multi-lobar disease or cavitary infiltrates.(17)
Although by definition CA-MRSA infections were centered in the community and not healthcare settings, CA-MRSA has been increasingly reported as the causative agent of nosocomial infections. This emergence makes unclear the main distinction between CA-MRSA and HA-MRSA; therefore, examination of the specific resistance patterns and possibly sophisticated molecular techniques may be required to differentiate the two. This distinction likely will have implications in treatment, surveillance and containment strategies in the hospital setting.
Treatment Options
Treating infections due to CA-MRSA requires understanding not only how these infections might present, but also awareness of resistance patterns in the organisms. As with treatment of other infections, a proper history and physical examination, followed by appropriate tests are all key in devising an initial empiric therapeutic plan. Because predicting organism sensitivities has become more difficult, sending a culture should be a routine part of the evaluation of any SSTI if there is any material to culture. When CA-MRSA is suspected, empiric therapy should be initiated before culture and sensitivity data can be known based on local susceptibility patterns.
A distinct antibiotic resistance pattern is one of the hallmarks of CA-MRSA, though as antibiotic use, and in some cases misuse, increases, resistance pattern may change. While many CA-MRSAs are resistant to the beta-lactam antibiotics used historically to treat SSTI, many isolates remain sensitive to a number of non beta-lactam antibiotics. These agents include trimethoprim-sulfamethoxazole (tmp-smx), clindamycin, fluoroquinolones, rifampin and the tetracyclines. In one large study, CA-MRSA isolates were 100% susceptible to tmp-smx, 95% susceptible to clindamycin, 92% susceptible to tetracycline, 60% susceptible to fluoroquinolones, but only 6% to erythromycin.(18) While a large percentage of isolates are susceptible to clindamycin, there is concern about inducible clindamycin resistance in strains resistant to erythromycin. This inducible resistance can be determined by microbiology laboratories by D-zone disk diffusion testing, a simple test which can be performed by most microbiology laboratories.
As with all infections, knowledge of the local resistance patterns, when available, is helpful. Choice of initial antibiotic therapy, when indicated, is dependent upon specific patient characteristics including drug allergies, prior antibiotic therapy and, in the case of recurrent infections, prior culture and in vitro sensitivity data. Often, tmp-smx or clindamycin are initial drugs of choice. It should be noted that tmp-smx may have limited activity against Streptococcus pyogenes which continues to be an important cause of cellulitis. GI-upset and diarrhea are common side-effects of clindamycin. Tetracyclines can cause photosensitivity and are teratogenic.
Because of relatively broad-spectrum antibacterial activity and ease of dosing, fluoroquinolones are often used in the treatment of purulent SSTI. However, fairly high rates of resistance to quinolone antibiotics in CA-MRSA and knowledge that fluoroquinolone monotherapy for S. aureus infections in general can lead to the development of resistance during treatment (with subsequent treatment failure) make these an unattractive first line choice. Fluoroquinolones are also associated with tendon rupture. Monotherapy with rifampin also has a high rate of failure in S. aureus infections, as a consequence of resistance developing during treatment, and should not be used. All antibiotics can cause significant allergic reactions; treatment with systemic antibiotics can lead to a change in body flora and development of super-infections including Clostridium difficile-associated disease.
Prospective and randomized data on the treatment of SSTI caused by CA-MRSA are lacking but two trials lend some insight. One study examined patients who required incision and drainage but not subsequent hospitalization for purulent SSTI.(19) Patients were randomized to tmp-smx DS or doxycycline therapy. No statistically significant difference in the rates of failure of treatment between the two groups was noted. While the study size was small, cure rates were high, suggesting that these and potentially other antibiotics to which CA-MRSA were susceptible should be considered as reasonable initial empiric therapeutic choices.
A second trial compared a beta-lactam antibiotic, cephalexin, to placebo after incision and drainage of SSTI.(19) No difference in treatment outcomes was observed in the two arms. While beta-lactam antibiotics are not active against CA-MRSA (which represented 87% of staphylococcus isolates in this study), high cure rates (84-90%) in both the cephalexin and placebo arms suggest that incision and drainage without subsequent systemic antibiotic therapy may be sufficient for the cure of many patients with purulent SSTI. Interestingly, this study contained a high percentage of patients with comorbid conditions, including intravenous drug abuse and HIV, representing an almost "worst case scenario" population, and still a high cure rate was observed.
While patients are frequently treated with both incision and drainage of purulent cutaneous abscesses followed by a course of systemic antibiotics, further prospective data may change this paradigm. Since use of antibiotics contributes to the development of resistant bacterial pathogens in general, and perhaps even specifically to the rise of the virulent CA-MRSA strains, an equivalent therapeutic strategy, which does not involve antibiotic use, would be attractive.
Nevertheless, with the current available data, the following strategy is reasonable. First, most importantly, purulent lesions should be completely incised and drained. As mentioned above, this intervention alone may be sufficient to cure SSTI due to CA-MRSA. Second, if there is extensive surrounding cellulitis, the selection of an antimicrobial agent with anti-CA-MRSA activity is important. Choice and dosing depend on patient characteristics including renal function, medicine allergies and potential for drug interactions. Reasonable choices include tmp-smx, in the form of tmp-smx DS, two tablets twice daily (one tablet twice daily is a urinary-tract infection dose and may not be sufficient to treat SSTI due to CA-MRSA), clindamycin (patients typically tolerate a dose of 450 mg three times daily), and doxycycline or minocycline (both at a dose of 100 mg twice daily).
In cases where streptococcal infection remains high in the differential diagnosis (e.g., simple cellulitis), but coverage for CA-MRSA is desired as well, clindamycin is a reasonable option, as it provides good anti-streptococcal coverage. If tmp-smx or doxycycline are used to cover for CA-MRSA, consideration should be given to adding a beta-lactam antibiotic (e.g., amoxicillin, cephalexin) for better streptococcal coverage.
There is some disagreement as to the appropriate antimicrobial therapy for pneumonia caused by CA-MRSA. Vancomycin and linezolid both have activity against MRSA in vitro. Vancomycin is a bactericidal drug, but penetrates lung tissue poorly and clinical treatment failures have been reported. While studies have compared the use of vancomycin versus linezolid for the treatment of MRSA pneumonia, it remains unclear which drug is superior.
In general, treatment of MRSA pneumonia is disappointing, with cure rates of approximately 65%. (21)(22) [These studies examined the treatment of nosocomial pneumonia likely caused by HA-MRSA. There are no data regarding treatment of CA-MRSA pneumonia.] Daptomycin is a newer antimicrobial agent with activity against MRSA. It is inactivated by lung surfactant and should not be used to treat pneumonia. It may have a role in treating complicated SSTI due to CA-MRSA, but as it is a last option to treat resistant infections due to vancomycin-resistant gram positive organisms, it should not be considered first line therapy where other antimicrobials have indications because of concerns for development of resistance.
Surveillance and Eradication Regimens
There is considerable interest in the prevention of recurrent CA-MRSA infections. Despite high cure rates, each individual infection does carry morbidity and recurrence is, unfortunately, a prominent component of the natural history of CA-MRSA SSTI. It is hypothesized that since infection occurs either from organisms colonizing the individual or by exposure of the individual to others with CA-MRSA carriage, decolonization may decrease the number of infections due to CA-MRSA.
Decolonization of carriage with intranasal mupirocin, a topical antimicrobial agent with activity against gram positive organisms, is often attempted. Studies show eradication is possible, especially in healthy subjects, but fail to show that these successes have any impact on disease recurrence.(23)
Chlorhexidine is a chemical antiseptic with microbicidal activity against both gram negative and gram positive bacteria including S. aureus. Several studies, including one prospective, randomized study, investigated its efficacy in decreasing colonization. Results from these studies have been somewhat disappointing but it remains a prominent part of most decolonization regimens.(24)
Another decolonization strategy employs oral antibiotic therapy. Several antibiotics and antibiotic combinations have been studied, typically using rifampin alone or in combination with other antimicrobials. One study examined the combination of rifampin + doxycycline along with mupirocin nasal ointment and chlorhexidine washes versus no treatment.(25) Decolonization was significantly more likely in the treatment arm of this study, though outcome data are somewhat lacking.
Finally it is often recommended that patients have close contacts screened and, if identified as carriers, have eradication attempts made. Laundry of affected individuals should be kept separate and washed in hot water when possible. Lotions, soaps, make-up and other intimate items should be discarded and replaced during a course of treatment of active lesions.
In patients with multiple recurrences, a course of suppressive antibiotic therapy is sometimes recommended. There are very little data guiding such a course of action but, for patients who have had multiple treatment courses and decolonization, it may be reasonable to institute a defined-length course of suppression. Selecting an antibiotic to which CA-MRSA is often susceptible and has not been previously used for treatment in the given patient in the past and treating for 4-6 weeks would be a somewhat typical strategy. Long-term suppression has not been studied. Patients may have recurrences while on suppressive therapy.
While studies investigating individual methods of decolonization have been either disappointing or conflicting, there are data suggesting that a standardized decolonization regimen may be effective.(26) It may be useful, therefore, to introduce an institution-wide decolonization routine, likely to involve mupirocin ointment and chlorhexidine washes along with heightened awareness of good hygiene.
Conclusion
CA-MRSA is a growing problem. It seems to possess a unique set of virulence factors which may explain its increased virulence and skin tropism. It is the causative agent of many purulent SSTI presenting from the community. It can cause deep and invasive infections. Recurrence is common. CA-MRSA is resistant in vivo to traditional anti-staphylococcal drugs of choice; SSTI due to CA-MRSA frequently require incision and drainage. Eradication of colonization with CA-MRSA remains an interesting theoretical therapeutic intervention.