Suppression of the Renin-Angiotensin-Aldosterone System
Course Authors
Samer Ellahham, M.D. and Helmy M. Siragy, M.D.
Dr. Ellahham is Head, The Center for Heart Failure, Department of Cardiac Sciences, SKMC/Cleveland Clinic Foundation, Abu Dhabi, UAE, and Dr. Siragy is Professor of Medicine and Endocrinology, Department of Medicine, and Director, Hypertension Center, The University of Virginia, Charlottesville, Virginia, USA.
Within the past 12 months, Dr. Ellahham reports no commercial conflicts of interest and Dr. Siragy reports no commercial conflicts of interest.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Describe the importance of blood pressure lowering for the prevention of cardiovascular complications
Discuss the role of the renin-angiotensin-Aldosterone system (RAS) in development of hypertension
Describe the mechanisms involved with different inhibitors of the RAS
Discuss the recent data of the blood pressure-lowering effects achieved through renin inhibition.
"It is not the answers that enlighten, but the questions." - Ionesco
Hypertension is a common disease that leads to significant cardiovascular morbidity and mortality worldwide. It is most common among African Americans, the elderly, diabetic and obese individuals.(1) Management of hypertension is critical in reducing end-organ complications such as stroke, heart failure, myocardial infarction or kidney disease. Despite the availability of numerous antihypertensive agents, several unmet goals remain such as adequate blood pressure control, greater protection against the development of organ damage, better treatment tolerability and adherence, and ultimately a more effective prevention of cardiovascular morbidity and mortality. These unmet goals are the reasons for the continued search for new antihypertensive drugs.
Current knowledge suggests that the renin-angiotensin-aldosterone system (RAS) is involved in the development of hypertension and its associated complications. Thus, inhibition of the RAS is a logical step for BP control and cardiovascular and renal protection.(2)> In this Cyberounds®, we will discuss the role of the RAS in the development of hypertension and the importance of renin in RAS activation, identify the mechanisms of different inhibitors of this system and summarize the emerging data that support the therapeutic potential of direct renin inhibition.
Current knowledge suggests that the renin-angiotensin-aldosterone system (RAS) is involved in the development of hypertension and its associated complications...
The Renin-Angiotensin System
The renin-angiotensin system (RAS) is a major hormonal autocrine/paracrine system that under normal conditions contributes to the regulation of cardiovascular and renal functions.(3) However, under pathologic condition this system is involved in mechanisms leading to enhanced vasoconstriction, sodium retention, inflammation, matrix formation and cellular hypertrophy.(4)(5)(6)

Figure 1. The Renin-Angiotension System.
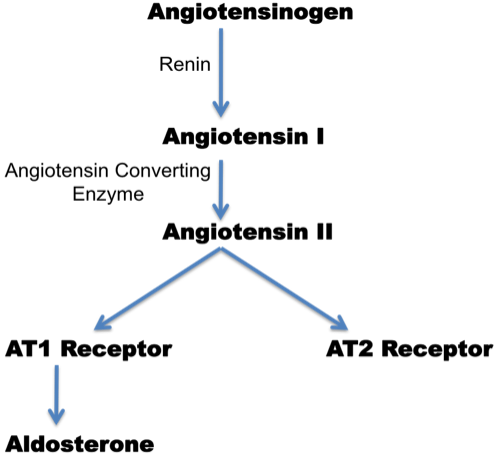

Angiotensin II (Ang II), the major effector peptide of the RAS, binds with equal affinity to two major receptors, the angiotensin subtype-1 (AT1) and subtype-2 (AT2) receptors. AT1 receptor is a member of the G protein-coupled receptor superfamily containing 7 trans-membrane domains and widely distributed on many cell types in Ang II targeted organs. This receptor has been identified in parts of the brain, adrenal glands, vasculature, heart and kidney. AT1 receptor mediates most of the established physiologic and pathologic effects of Ang II. These include actions on the cardiovascular system (vasoconstriction, increased blood pressure, increased cardiac contractility, vascular and cardiac hypertrophy), kidney (renal tubular sodium reabsorption and inhibition of renin release), increasing sympathetic nervous system activity and stimulation of aldosterone synthesis.(7) The AT1 receptor also mediates effects of Ang II on cell growth and proliferation, inflammation, and oxidative stress.
Ang II, via the AT1 receptor, also stimulates the production of aldosterone by the zona glomerulosa, the outermost zone of the adrenal cortex. Aldosterone is a significant regulator of sodium and potassium balance and thus plays a prominent role in regulating extracellular volume. It enhances the reabsorption of sodium and water in the distal tubules and collecting ducts and thereby promotes potassium excretion. It is also important to note that Ang II is a major trophic factor for the zona glomerulosa.(7)(8) In addition, stimulation of AT1 receptors in blood vessels promotes the release of endothelin, a potent vasoconstrictor hormone, causing elevation of BP.
AT2 receptor is highly expressed in fetal tissue. However, its expression decreases rapidly after birth. Although AT2 receptor is generally expressed in low copy in the adults, it is re-expressed in response to injury or stress. Under normal conditions, the net effect of Ang II usually produces a balance between AT1 and AT2 receptors. However, AT1 receptor is more highly expressed than the AT2 receptor in most tissues. The AT2 receptor is often upregulated by tissue injury and/or disease processes, making it more likely to be involved in tissue repair. In general, the AT2 receptor inhibits growth and cell proliferation in many tissues, thereby opposing the effects mediated by the AT1 receptor. In the cardiovascular system, the AT2 receptor was demonstrated to mediate a vasodilator cascade composed of bradykinin, nitric oxide and guanosine cyclic 3', 5'- monophosphate.(9)(10)(11)(12) Early studies strongly suggested that the AT2 receptor mediates vasodilation that is counter-regulatory to AT1 receptor-induced vasoconstriction.(13)
Dysregulation and Hypertension
Dysregulation of the RAS is involved in the pathogenesis of hypertension. Historically, RAS contribution to the development of hypertension has been attributed to its increased activity in systemic circulation, while the potential contributions of its tissue component remains poorly defined.(7) In addition to Ang II involvement in secondary forms of hypertension such as renovascular hypertension, there is evidence that RAS is also involved in essential hypertension. While plasma renin activity (PRA) levels may be elevated in renovascular hypertension, its levels vary widely in patients with "essential" hypertension.
The majority of patients with essential hypertension have PRA within the "normal" range, although it has been argued that a normal renin level in the face of hypertension may be inappropriate. Since renin is the rate-limiting step in Ang II formation, it becomes clear that maneuvers that regulate its activity may contribute to BP regulation. Some patients with hypertension such as African Americans may present, however, with low PRA. Those individuals may still benefit from RAS blockade, although at higher doses. This finding suggests that the circulating levels of PRA might not necessarily reflect tissue activities of the RAS system. This is particularly evident with regard to the kidney, with several lines of evidence pointing to substantial involvement of intrarenal Ang II in progression of renal damage, despite low circulating levels of renin and Ang II.
The RAS also plays a pivotal role in several nonhypertensive conditions, particularly congestive heart failure (CHF), myocardial infarction and kidney diseases such as diabetic nephropathy...
Other Consequences of Dysregulation
The RAS also plays a pivotal role in several nonhypertensive conditions, particularly congestive heart failure (CHF), myocardial infarction and kidney diseases such as diabetic nephropathy. In these conditions, the activity of tissue component of the RAS is increased. In heart failure, the contribution of Ang II to increased peripheral vascular resistance (cardiac afterload) also plays a major role in progressive ventricular dysfunction. Beyond progression of renal disease, there is additional clear evidence of involvement of Ang II in the development of both vascular and cardiac hypertrophy and remodeling, as well as vascular damage and atherosclerosis, effects that appear to have significant impact on morbidity and mortality.
Ang II can also increase intravascular volume by acting centrally to stimulate thirst drive and to enhance the release of antidiuretic hormone. Additionally, Ang II facilitates the release of norepinephrine from peripheral nerve terminals, as well as from the central nervous system, thereby increasing myocardial contractility and the release of additional renin. Ang II is also known to stimulate cell proliferation, left ventricular hypertrophy, vascular media hypertrophy, neointima formation in atherosclerosis and nephrosclerosis by activation of the AT1 receptor. Transgenic mice overexpressing the human AT1 receptor in cardiomyocytes developed cardiac hypertrophy and interstitial collagen deposition without alterations in BP and died prematurely of heart failure.
Similarly, Ang II plays an integral role in the pathogenesis of hypertension-induced end-organ damage. It has been demonstrated that Ang II causes monocyte recruitment and a vascular inflammatory response in the kidney, with both processes completely prevented by AT1 receptor antagonism. Overall, Ang II has a wide variety of hemodynamic and cellular actions mediated though the AT1 receptor, including the capacity to elevate systemic BP and to promote cardiac hypertrophy and end-organ injury. Thus, the regulation of Ang II activity at the AT1 receptor could affect both BP control and reduce/prevent cardiovascular pathology.
Angiotensin Converting Enzyme Inhibition
Angiotensin converting enzyme inhibitors (ACEIs) competitively block the action of ACE and thus the conversion of Angiotensin I (Ang I) to Ang II, thereby reducing levels of Ang II. ACEIs also decrease aldosterone and vasopressin secretion and sympathetic nerve activity. Reduction in Ang II synthesis by ACEIs is associated with increased renin production due to inhibition of the negative feedback mechanism. Thus, with the increase in renin secretion during ACEI therapy, there is significant increase of Ang I levels that tend to partially overcome the ACE blockade. This would be especially likely in patients with high-renin levels such as in the presence of volume-depletion or diuretic use. ACEIs also prevent degradation of bradykinin and lead to accumulation of this peptide. This effect is associated with increased bradykinin-dependent release of NO and vasoactive prostaglandins, including prostacyclin and prostaglandin E2. These actions may potentially contribute to the vasodilatory, antithrombotic, antiatherogenic and antiproliferative effects of ACEIs.(14)(15)
ACEIs, when used in medium to large doses, achieve satisfactory blood pressure control...
ACEIs, when used in medium to large doses, achieve satisfactory blood pressure control. In addition, ACEIs improve cardiac hypertrophy, heart failure, post myocardial infarction remodeling and slow down the progression of kidney diseases. Results of the Heart Outcomes Prevention Evaluation (HOPE) trial and other studies indicate broad cardiovascular benefits of ACEI therapy in "high-risk" patients including both hypertensive and normotensive individuals. It is possible that these benefits occur in part independently of their blood pressure-lowering effect via reduction in Ang II formation. Several large-scale studies showed reduction in incidence of new-onset diabetes in association with ACEI therapy. This has been shown with captopril in patients with hypertension (CAPP), with ramipril in patients at high risk for cardiovascular disease (HOPE), with enalapril in Studies Of Left Ventricular Dysfunction (SOLVD) and with trandolapril in patients with stable coronary disease [Prevention of Events with ACE Inhibition (PEACE)]. The mechanism of this benefit has not been fully elucidated. Interestingly, the recently published PEACE trial did not demonstrate a benefit of the ACE inhibitor trandolapril in reducing cardiovascular morbidity and mortality in patients with stable coronary disease and preserved left ventricular systolic function.
The results of PEACE are in contrast to prior studies performed in patients with coronary artery disease, such as HOPE and EURopean trial On reduction of cardiac events with Perindopril in stable coronary Artery disease (EUROPA). The PEACE trial did not demonstrate a benefit of the ACE inhibitor trandolapril in reducing cardiovascular morbidity and mortality in patients with stable coronary disease and preserved left ventricular systolic function. As reported by the PEACE investigators, this discrepancy might be explained by the inclusion of lower-risk patients who received effective concomitant therapy such as coronary revascularizations and statin treatment. However, both cardiovascular morbidity and mortality rates were similar in EUROPA and PEACE. In addition, the percentages of patients receiving antiplatelet agents, beta blockers and lipid-lowering agents were similar in EUROPA and PEACE.(16)(17)(18)(19)(20)(21)
ACEI therapy is generally well tolerated by most patients but is nonetheless associated with some significant side effects. Most frequent among these is dry cough, which has been attributed to accumulation of substance P, which is normally degraded by kininase II. More serious side effects common to ACEIs include angioedema and fetal abnormalities. Other consequences of ACE inhibition may include hypotension, hyperkalemia and deterioration of renal function.(22)
Angiotensin Receptor Blockers (ARBs)
The AT1 receptor mediates most of the known actions of Ang II that contribute to hypertension and volume dysregulation (vascular smooth muscle contraction, aldosterone secretion, renal sodium reabsorption, pressor and tachycardia responses) as well as to cardiovascular damage (cellular hypertrophy or proliferation, prothrombotic and proinflammatory effects, and superoxide formation). Several ARBs have been synthesized, including losartan, valsartan, irbesartan, candesartan, eprosartan, telmisartan and olmesartan.(23)
Since ARBs act by blocking Ang II action at the receptor level, rather than by inhibiting its synthesis, they antagonize AT1-mediated effects of Ang II no matter how it is synthesized. Thus, if there is significant Ang II synthesis by enzymes other than ACE (alternate pathways) such as chymase, ARBs are still able to inhibit AT1 receptor.(24) In contrast to the ACEIs, ARB therapy results in an increase in Ang II levels.(25) As with ACE inhibition, blockade of the AT1 receptor inhibits the negative feedback loop, leading to increased renin secretion and thus to increased synthesis of Ang I. In the case of ARBs, the increase in Ang I causes an increase in Ang II, which is freely able to bind to AT2. Like the ACEIs, ARBs reduce blood pressure by decreasing systemic vascular resistance.
Reduced systemic vascular resistance results from a combination of inhibition of Ang II-mediated vasoconstriction, reduced sympathetic nervous system activity and reduced extracellular volume.(11) ARB therapy has also been shown to reduce markers of inflammation, suggesting an anti-inflammatory effect, and to reverse endothelial dysfunction, indicating the possibility of significant antiatherogenic effects.(26)(27)(28) In patients with hypertension, ARB therapy has also been shown to improve arterial compliance and vascular wall remodeling independent of the blood pressure-lowering effect. This suggests that ARB therapy may contribute to reversal of vascular wall damage.(29)
Several large trials support the idea that the ARB class may confer benefits on target organ protection beyond the lowering of blood pressure per se. In patients with hypertension and left ventricular hypertrophy, ARB-based therapy, compared with beta-blocker (atenolol)-based therapy with identical blood pressure control, has been shown to significantly reduce the composite risk of cardiovascular death, stroke, MI and to decrease the rate of new-onset diabetes [Losartan Intervention For Endpoint reduction in hypertension (LIFE) study].(30)
ARB therapy has also been shown to improve arterial compliance and vascular wall remodeling independent of the blood pressure-lowering effect...
Similarly, ARB-based therapeutic regimens, compared with conventional therapy, have been shown to reduce the progression of nephropathy in patients with diabetic nephropathy. Nephropathy develops in about 40% of patients with type 2 diabetes. Microalbuminuria, the earliest clinical evidence of diabetic nephropathy, is associated with a ten-fold increase in the risk of progression to overt nephropathy and eventual end stage renal disease. More than 50% of patients starting dialysis are type 2 diabetics.
Two major studies - the Reduction of Endpoints in NIDDM with the Angiotensin II Antagonist Losartan (RENAAL) Study and the Irbesartan in Diabetic Nephropathy Trial (IDNT) - showed that ARBs are effective in reducing the progression of renal disease in patients with type 2 diabetes and high BP. Standard care for diabetes was maintained. Using conventional high BP therapy such as diuretics, beta-blockers, and calcium channel blockers (but no ACE inhibitors or other ARBs), BP control was similar in the placebo and ARB-treated groups.
Thus, the concept has emerged from these two trials that ARBs protect the kidney independent of BP reduction.(31)(32) Although BP control was similar in all groups, the incidence of the primary composite endpoint was significantly lower in the irbesartan cohort compared with either the amlodipine or placebo groups. The angiotensin II receptor antagonist, irbesartan, had better renal outcomes than amlodipine, placebo and non-study antihypertensive agents. A significant increase in the time to a doubling of the serum creatinine concentration, a measure that approximates a 50% reduction of the glomerular filtration rate, indicates a slowing of the rate of progression of nephropathy. Also, the mean increase in the serum creatinine and the mean decrease in creatinine clearance were significantly slower in the irbesartan group. The patients in the amlodipine group had worse renal outcomes than those in the irbesartan group, although there was equal control of blood pressure in the amlodipine group. Irbesartan produced an important delay in the progression of renal disease. Proteinuria was reduced on average by 33% as compared with 6% in the amlodipine group and 10% in the placebo group. These reductions in proteinuria were maintained throughout the follow-up period.
There were no significant differences among the treatment groups in the secondary cardiovascular endpoint. The secondary cardiovascular endpoint was the composite of death from cardiovascular causes, nonfatal myocardial infarction, heart failure resulting in hospitalization, a permanent neurological deficit caused by a cerebrovascular event or lower limb amputation above the ankle. Of interest, the rate of heart failure requiring hospitalization in the irbesartan-treated patients was 23% lower than in patients assigned to placebo. Also, the patients assigned to receive amlodipine had a rate of nonfatal myocardial infarction that was 41% lower than patients assigned to placebo.(31)
Like ACEIs, the use of ARBs is contraindicated in pregnant women because of the association of increased fetal morbidity and mortality, particularly with exposure during the second and third trimester. ARB therapy is otherwise generally well tolerated, even in many patients who discontinue ACEI therapy because of side effects. Rare reports of angioedema and cough have emerged from the early premarketing clinical trials with ARBs.
Direct Renin Inhibitors In Patients With Hypertension
The most recent class of agents that block the RAS to be introduced is the direct renin inhibitors represented by aliskiren, which was recently approved for treatment of hypertension. This compound differs from the ACEIs and ARBs in that, by blocking the catalytic activity of renin at the point of activation of the RAS, it blocks the synthesis of all angiotensin peptides and prevents the compensatory increase in renin activity.
Aliskiren
Aliskiren, a novel direct renin inhibitor, lowers blood pressure by decreasing renin activity, and angiotensin I and II levels.(33)(34) Studies done in patients with hypertension showed that aliskiren reduced systolic blood pressure by 12-15 mm Hg and diastolic blood pressure by 2-12 mm Hg. Its efficacy was comparable to losartan 100 mg, valsartan 80-320 mg and irbesartan 150 mg. When used in combination with ramipril, valsartan or hydrochlorothiazide, it provides additional BP reduction compared with placebo or monotherapy.
Pharmacology
Renin is a protease enzyme secreted by the juxtaglomerular cells in the kidney in response to sodium depletion, reduced blood volume and renal perfusion, or increased sympathetic central nervous system activity. Renin cleaves angiotensinogen to form Ang I, which is then converted to Ang II by ACE and non-ACE pathways. Increased Ang II level through a negative feedback loop inhibits the release of renin. As a result of this negative feedback mechanism, renin becomes the major rate limiting step of RAS activity and a main target for inhibiting Ang-II-related effects.
Aliskiren is the first known agent in a new class of nonpeptide, low-molecular-weight, orally active renin inhibitors designed through a combination of molecular modeling techniques and crystal structure elucidation. It binds with high specificity to the proteolytic active sites of renin. It exhibits oral bioavailability and an extended half-life, allowing convenient once daily oral administration.(35)(36)(37)
Aliskiren, a novel direct renin inhibitor, lowers blood pressure by decreasing renin activity, and angiotensin I and II levels.....

Figure 2. Consequences of Inhibition of Renin Activity.
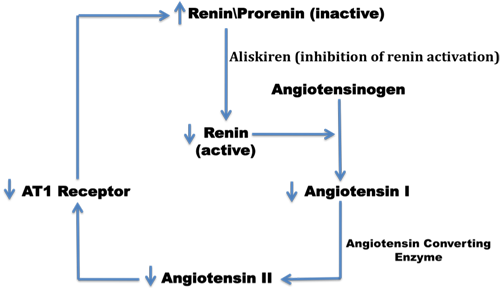

Aliskiren lowers BP by inhibiting renin activity and, therefore, decreasing the circulating levels of Ang I and Ang II, increasing renin release and decreasing urinary aldosterone excretion.(38)(39)
Pharmacokinetics
Aliskiren reaches the peak plasma concentration within 1-6 hours after oral administration. Aliskiren has a half-life of approximately 24 hours; thus, steady-state blood levels can be expected in about 5-8 days. No dose adjustments are needed in individuals with mild to moderate hepatic or renal insufficiency or in the elderly. Aliskiren is metabolized by cytochrome P450 enzyme 3A4.(40)(41)(42)(43)(44)(45)
Clinical Trials
The efficacy and safety of aliskiren have been demonstrated in several randomized, double-blind, placebo-controlled clinical trials in patients with mild-to-moderate hypertension. Approximately 2730 patients received aliskiren at various dose ranges.
Aliskiren v. ARBs
Stanton et al. compared aliskiren to losartan in 197 patients with mild to moderate hypertension in a randomized double-blind trial. Enrolled patients were randomized to receive aliskiren 37.5, 75, 150, 300 mg or losartan 100 mg daily for 4 weeks. Results showed that aliskiren 150 or 300 mg daily provided similar efficacy compared with losartan 100 mg daily with systolic blood pressure (SBP) reductions of 10-14 mm Hg and diastolic blood pressure reductions of 2-8 mm Hg. Plasma renin activity (PRA) was reduced by 77% and 83% for aliskiren 150 and 300 mg groups, respectively, and increased by 110% for losartan group. Aliskiren was well tolerated with the most common adverse effects being fatigue, weakness, gastrointestinal complaints, and headache.(45).
In another randomized, multicenter, double-blind trial, Gradman et al. compared aliskiren to placebo and irbesartan in 652 adult patients with mild-to-moderate hypertension. After a 2-week, single-blind placebo run-in, patients were randomized to receive aliskiren 150, 300, or 600 mg, irbesartan 150 mg or placebo daily for 8 weeks. Results showed a dose-related antihypertensive effect of aliskiren up to 300 mg daily. Aliskiren 150 mg daily provided similar efficacy to irbesartan 150 mg daily. Placebo and irbesartan provided blood pressure reductions of 5.3/6.3 mm Hg and 12.5/8.9 mm Hg, respectively.
Compared with placebo, aliskiren further reduced SBP by 6.1, 10.5 and 10.4 mm Hg, and diastolic blood pressure (DBP) by 2.9, 5.4 and 5.1 mm Hg in aliskiren 150, 300 and 600 mg groups, respectively (all P values vs. placebo significant). Compared with irbesartan, aliskiren further reduced SBP by 3.3 and 3.2 mm Hg, as well as DBP by 2.9 and 2.6 mm Hg in aliskiren 300 and 600 mg groups, respectively (P values vs. irbesartan significant for DBP measures only). More patients receiving aliskiren 300 and 600 mg achieved blood pressure control compared with those receiving placebo and irbesartan (38-50% aliskiren, 34% irbesartan, 21% placebo; P<0.05 for both comparisons). The most common adverse effects reported were nervous system symptoms (6.3-10.0% aliskiren, 9.2% placebo and 6.7% irbesartan) and gastrointestinal symptoms (3.9-9.2% aliskiren vs. 3.8% placebo and 6.7% irbesartan).(46)
Aliskiren Combined With a Diuretic
In a multicenter, randomized, double-blind, 8-week trial, Villamil et al. compared aliskiren, HCTZ, aliskiren/HCTZ combination and placebo in hypertensive patients (DBP 95-109 mm Hg). A total of 2776 patients were randomized to receive aliskiren 75, 150 or 300 mg daily, HCTZ 6.25, 12.5 or 25 mg daily, aliskiren and HCTZ combination or placebo. Compared with placebo, aliskiren 300 mg provided an additional blood pressure reduction of 8.2/3.4 mm Hg (P<0.0001). HCTZ 25 mg provided an additional blood pressure reduction of 6.8/2.5 mm Hg (P<0.05). Aliskiren/HCTZ 300/25 mg provided an additional reduction of 13.7/7.4 mm Hg (P<0.0001 vs. placebo; P<0.05 vs. each component monotherapies). Rates of adverse effects were similar among groups (HCTZ up to 11%; aliskiren up to 9.8%; aliskiren/HCTZ up to 16.6%; and placebo 8.8%). Hypokalemia occurred more frequently in the HCTZ group (0.6-5.2% vs. 0-1.5% aliskiren; 0.7%-3.4% aliskiren/HCTZ; and 1.3% placebo). Discontinuation rates due to adverse effects occurred more frequently in aliskiren 300 mg group (4.4% vs. 3.6% placebo).(47)
Aliskiren Combined With Other RAS Inhibitors
Azizi et al. assessed the effects of aliskiren-valsartan combination in 12 healthy, mildly sodium-depleted, normotensive male volunteers, who received single doses of aliskiren 300 mg, valsartan 160 mg, aliskiren/valsartan 150/80 mg and placebo. The reductions in mean arterial pressure at 4 hours post dose were 6.6, 4.9 and 7.4 mm Hg for valsartan 160 mg, aliskiren 300 mg and aliskiren/valsartan 150/80 mg, respectively. At 24 hours, the results were 2.4, 1.2 and 1.0 mm Hg, respectively. At 48 hours, the results were increases of 2.6 and 0.8 mm Hg, for aliskiren and aliskiren/valsartan, respectively, and a reduction of 0.9 mm Hg for valsartan.(38)
In a multicenter, randomized trial, Pool et al. randomized 1123 patients to receive aliskiren 75, 150 or 300 mg, valsartan 80, 160 or 320 mg, aliskiren/valsartan 75/80, 150/160 and 300/320 mg, valsartan/hydrochlorothiazide (HCTZ) 160/12.5 mg or placebo daily. At week 8, placebo reduced blood pressure by 10.0/8.6 mm Hg; aliskiren groups reduced SBP by 12.1 to 15.0 mm Hg and DBP by 10.3 to 12.3 mm Hg; valsartan groups reduced SBP by 11.2 to 16.5 mm Hg and DBP by 10.5 to 11.3 mm Hg. All three aliskiren-valsartan combinations significantly lowered blood pressure compared with placebo. Aliskiren-valsartan 150/160 and 300/320 mg have similar efficacy compared with valsartan/HCTZ 160/12.5 mg. The most commonly reported adverse effects among all 1125 randomized patients were headache (5.7%), fatigue (2.8%), back pain (1.8%) and diarrhea (1.6%).(48)
The Aliskiren in the eValuation of prOteinuria In Diabetes (AVOID) trial is a randomized, multinational, double-blind controlled trial offering additional evidence for the benefit of combination therapy for better suppression of the RAS. The study was performed in 599 patients with type 2 diabetes mellitus (DM), hypertension and diabetic nephropathy [excluding patients with hyperkalemia, estimated glomerular filtration rate (eGFR) <30 or proteinuria >3500mg/day] who had well-controlled hypertension and were already receiving maximum renoprotective doses of losartan (100 mg daily). Patients were randomized to receive either the combination of aliskiren plus losartan versus losartan plus placebo for six months. The combination group experienced a 20% reduction in albuminuria. There was only a small reduction in BP in the combination group (2 mm Hg lower systolic BP and 1 mm Hg lower diastolic BP). Adverse events were similar in the two groups. The study, however, used a surrogate endpoint and only followed up patients for a relatively short period of time. Furthermore, the results of this study can only be applied to patients with diabetic nephropathy who have an eGFR>30 and normal serum potassium.
More studies are needed to determine the safety and efficacy of such a therapeutic regimen over a longer period of time and to assess hard clinical endpoints, especially after the results of the recently released the ONgoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET) study.(2)(49)(50)(51) The investigators discussed several study limitations, noting the possibility that changes in antihypertensive medications after randomization may have at least partially skewed the study's results. Furthermore, they pointed out that there is currently no evidence that aliskiren renal protective effect is sustained over longer time periods, emphasizing that long-term studies must be conducted to elucidate whether the beneficial effect on the kidney that is seen in the short term is sustained.
The Aliskiren Observation of Heart Failure Treatment (ALOFT) trial studied the effects of adding the direct renin inhibitor aliskiren to an ACE inhibitor in patients with heart failure. Patients with New York Heart Association class II to IV heart failure, current or past history of hypertension and plasma brain natriuretic peptide (BNP) concentration >100 pg/mL who had been treated with an ACE inhibitor (or angiotensin receptor blocker) and ß-blocker were randomized to 3 months of treatment with placebo (n=146) or aliskiren 150 mg/d (n=156). The primary efficacy outcome was the between-treatment difference in N-terminal pro-BNP (NT-proBNP). Patients' mean age was 68 years, mean ejection fraction was 31% and mean±SD systolic blood pressure was 129±17.4 mm Hg. Sixty-two percent of the patients were in New York Heart Association functional class II and 33% were taking an aldosterone antagonist.
Plasma NT-proBNP rose by 762±6123 pg/mL with placebo and fell by 244±2025 pg/mL with aliskiren (P=0.0106). BNP and urinary (but not plasma) aldosterone were also reduced by aliskiren. Clinically important differences in blood pressure and biochemistry were not seen between aliskiren and placebo. The study concluded that the addition of aliskiren to an ACE inhibitor (or angiotensin receptor blocker) and ß-blocker had favorable neurohumoral effects in heart failure and appeared to be well tolerated.(52)
Pharmacological blockade of the RAS is currently the cornerstone of therapy for diabetic nephropathy...
Pharmacological blockade of the RAS is currently the cornerstone of therapy for diabetic nephropathy. More complete inhibition of this system can be achieved by using combination regimens that employ medications with different/complementary mechanisms of action. The novel direct renin inhibitor aliskiren provides a more complete inhibition of the RAS by not allowing the secondary rise in plasma renin activity after the administration of an ACE inhibitor or an angiotensin receptor blocker. Hence, combining aliskiren with one (or both) of these agents offers the potential for a more complete blockade of the RAS. This study offers support to the notion that a more complete blockade of RAS leads to a better reduction in proteinuria, possibly through an effect that is not mediated by blood pressure (BP) reduction.
Conclusions
Systemic hypertension is a major risk factor for coronary disease, stroke, renal and heart failure, the leading causes of morbidity and mortality in the United States. The renin-angiotensin-aldosterone system has been a major target site for many antihypertensive agents, including ACEIs, ARBs and direct renin inhibitors. ACEIs block the conversion of Ang I to Ang II. ARBs selectively block the binding of Ang II to the AT1 receptors. Aliskiren, a novel direct renin inhibitor, lowers blood pressure by decreasing renin activity, angiotensin I and II levels. Therapeutic approaches that target more complete inhibition of the RAS may offer additional clinical benefits for patients with hypertension. These approaches may include dual blockade using ACEIs or ARBs, and aliskiren. Adding a diuretic to the RAS blockade could also produce further reduction in blood pressure in patients not achieving the goal on monotherapy.