Course Authors
Hanyi Zhuang, Ph.D., and Hiroaki Matsunami, Ph.D.
Dr. Matsunami is assistant professor, Department of Molecular Genetics and Microbiology, Duke University School of Medicine, Durham, NC, and Dr. Zhuang is on the faculty of the Department of Pathophysiology, Shanghai Jiaotong University School of Medicine, Shanghai 200025, PRC.
Within the past 12 months, Dr. Matsunami reports no commercial conflicts of interest and Dr. Zhuang reports no commercial conflicts of interest
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the signaling cascade following odorant receptor activation by odorant molecule
Compare and contrast the odorant receptor repertoires in representative mammalian species
Discuss the role of genetic variation in human olfactory perception.
The ability to smell is essential for animals to detect food sources, predators, mating partners and toxic compounds in the environment. In humans, the sense of smell is part of a variety of daily activities such as enjoying meals, detecting gas leakage, and even appreciating the body odor of others. Indeed, the mammalian olfactory system can detect and discriminate thousands of volatile odorants. Evolution has shaped this percept to exquisitely distinguish minute differences in the structure of odorant chemicals. For example, one can distinguish straight carbon chain aliphatic odorants with different carbon chain length or ones with same carbon chain length but with different functional groups. In addition, the olfactory system may perceive the same odorant molecules as having a different odor quality at various concentrations such as some perfumery components that can elicit a rather unpleasant smell at high concentrations.
Mammalian Odorant Receptors
Olfaction is determined by the odorant receptors (ORs) in the olfactory sensory neurons (OSNs) of the main olfactory epithelium. Mammalian ORs are G protein-coupled receptors (GPCRs) that are expressed on the cell-surface membrane at the tip of the dendrites in the OSNs. ORs, first cloned in 1991 by Buck and Axel, comprise the largest gene family among all GPCRs.(1)(2)(3)(4)(5) Approximately 400 genes are annotated as ORs in humans, whereas more than 1000 genes encode distinct ORs in mice, which are responsible for the detection of thousands of odors in the environment.(6)(7)(8)(9)(10)
Similar to other GPCRs, ORs are seven-transmembrane receptors that transduce signals from extracellular ligands by activation through guanine nucleotide-binding proteins, or G proteins, which in turn initiate a second-messenger signaling cascade to convert the odor binding event to the depolarization of the OSN. While the intracellular (IC) domains are relatively conserved, transmembrane domains of ORs are divergent among members.
It is thought that combinations of transmembrane domains may form a binding pocket to bind structurally diverse odorants. In fact, some of the transmembrane regions in other GPCRs such as the β-adrenergic receptor are also implicated in ligand binding.(11)(12)(13)(14) Like other GPCRs, it is likely that the ORs couple to the heterotrimeric G proteins. Gαolf is a stimulatory G protein that specifically mediates olfactory signals by activating the downstream adenylyl cyclase type III (ACIII), causing an increase in cyclic AMP (cAMP), which in turn opens cyclic nucleotide gated channels (CNG), leading to depolarization of the OSN (Figure 1). In addition, a cAMP-independent signal transduction pathway that involves inositol triphosphate (IP3) may play a minor role in olfactory signal transduction.(15)(16)

Figure 1. A Pathway of Olfactory Signal Transduction.
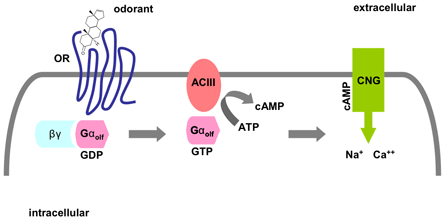
The binding of an odorant molecule to an odorant receptor (OR) leads to the interaction of the receptor with a GTP-binding protein Golf. This interaction in turn leads to the release of a GTP-coupled Gαolf subunit, which then stimulates adenylyl cyclase III (ACIII) to produce elevated levels of cyclic AMP (cAMP). The increase in cyclic AMP opens cyclic nucleotide-gated (CNG) cation channels, thus causing an alteration in membrane potential.

In situ hybridization analysis using OR probes revealed that there are four or more spatial zones, some are overlapping, within the olfactory epithelium that are marked by differential expression of ORs.(17)(18)(19)(20) OSNs that express a given OR are distributed randomly in one of the zones in the olfactory epithelium. It was also shown that different OSNs express different ORs.(21)(22)(23)(24) Since about 0.1% of OSNs express one given ORs out of the approximately 1000 ORs in the mouse, it was suggested that each OSN may express only one receptor gene. Single-cell reverse transcriptase polymerase chain reaction (RT-PCR) experiments supported this "one neuron-one OR" hypothesis.(21) Precisely speaking, a single OSN expresses only one allele of an OR gene. Though the mechanisms of this OR gene choice remain to be elucidated, it was found that negative feedback originating from OR coding sequences ensures the mutually exclusive OR expression; deletion or mutation of the coding region of an expressed OR gene allows a second OR gene to be expressed.(25)(26)(27)(28)
Classification of Odorant Receptors
ORs are encoded by the largest gene family in mammalian genomes and are undergoing rapid evolutionary change.(9)(29)(30) It is known that OR genes are highly divergent. The extensive variability of the ORs is consistent with the ability of animals to recognize chemicals of diverse structures. Among species, there are also dramatic differences in the size of the OR gene family. For example, while the mouse and rat have over 1000 OR genes, humans have fewer than 400 intact OR genes. Previous studies on the evolutionary genomics of mammalian OR repertoires investigated the evolutionary relationships between ORs located in clusters in human, mouse and rat genomes(31)(32)(33)(34)(35) to identify orthologous gene clusters,(36)(37)(38) orthologous receptors(36)(37)(39)(40)(41) and OR pseudogenes.(41)(42)(43) These efforts eventually led to the establishment of a standard system for OR classification.(44)
A survey of the genome sequences of 24 vertebrates species showed a clear separation of ORs into two classes: class I ORs and class II tetrapod ORs.(44) While a vast majority of ORs in fish belongs to class I ORs, a majority of the mammalian OR genes belongs to class II, while the rest are class I ORs.(44) This suggests that some ancient fish ORs are maintained and possibly expanded in mammals. It has been postulated that class I ORs in mammals might have evolved to recognize water-soluble hydrophilic compounds, while class II ORs are for hydrophobic compounds.(6) Recently, a set of experiments using mutant mice with OSNs ablated in a specific area of the olfactory epithelium showed that class I receptor activation may be important in mediating innate avoidance behavior.(45) The divergence of the OR genomes of fishes and tetrapods is probably the result of adaptive processes so that mammals, considering the change in their living environments, could detect a larger variety of molecules.
Evolution of the Human Odorant Receptor Repertoire
The human OR repertoire is undergoing a degeneration process in terms of the dramatic reduction in the number of intact OR genes, as mice have 2.7 times as many functional OR genes as humans. Approximately 60% of the more than 1000 OR genes are pseudogenes coding for a non-functional OR.(29)(46) In-depth comparative genome studies of mice and human revealed that the overall structures of the two OR repertoires are similar, covering a similar "receptor space" and possibly the same range of sequence motifs.(6)(8)(47)
Compared to other primate species, the human genome contains a higher percentage of OR pseudogenes than other mammalian species including chimpanzee, its closest primate relative.(48) Notably, the percentage of pseudogenes is estimated at 31% for 11 primate species and 17% in eight other primate species and this is compared with the approximated 20% in mice.(6)(43) It has been suggested that even though the number of ORs are greatly reduced in the human lineage, humans have probably retained the ability to recognize a broad but a less discriminating spectrum of chemicals.(6)(8)
Further studies across primate olfactory genomes confirm the attrition of the intact human OR repertoire mainly caused by pseudogenization.(41)(42) The most recent of these disruptions still segregates with the intact form, resulting in population polymorphisms that can be readily sampled.(49)(50) 26 of these segregating ORs have been identified.(49)(51)(52) These results demonstrated the presence of segregating pseudogenes that can potentially contribute to the rapid degeneration of the human olfactory system as well as the extensive variability in human odor perception.Odorant Information Coding in the Olfactory Bulb
How does the brain process the olfactory sensory information that comes from OSNs, each of which expresses one out of more than 1000 different ORs? Each OSN sends its axon to a brain area called the olfactory bulb (OB) and terminates in a ball-like structure called the glomerulus, which is just beneath the surface of the OB. Since each OB contains about 1800 glomeruli in the mouse(53) and receives input from two to four million OSNs,(54)(55) each glomerulus may receive approximately 1000 to 2000 olfactory axons.(56) As trace amounts of OR mRNAs can be detected in the axons of OSNs, in situ hybridization studies using OR probes were carried out in the OB to analyze the targeting of olfactory axons.(56)(57) In addition, using homologous recombination, gene "knock-in" mice where tau-lacZ marker gene was inserted downstream to a specific OR gene were generated to observe single axons that originated from OSNs expressing a given OR.(58)(59)
These two types of experiments have revealed that the axons of neurons expressing the same receptor converge onto a few glomeruli (Figure 2). Remarkably, the axons of neurons expressing a given OR target glomeruli at very similar locations in different animals, though the precise location of a particular glomerulus may not be fixed.(60)(61) At least in some cases, neurons that express receptors that have high similarity to each other target neighboring glomeruli.(59)(62)(63)(64)

Figure 1. Expression of Odorant Receptors and Targeting of Olfactory Axons in Mammals.
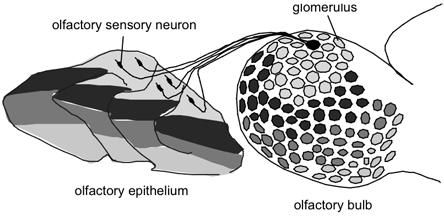
Olfactory sensory neurons that express a given odorant receptor distribute randomly in one of the four or more spatial zones in the olfactory epithelium. Olfactory sensory neurons that express a given odorant receptor converge onto a few glomeruli in the olfactory bulb. Since one neuron is likely to express only one odorant receptor gene, each glomerulus may receive inputs derived from one type of odorant receptor.

Because each glomerulus seems to receive axons originating from OSNs that express the same receptor, activation of olfactory axon terminals in a glomerulus that is generated by odorant stimulation is likely to reflect activation of one type of OR. Glomeruli or subregions in the OB activated by odorants have been mapped by various methods including intrinsic imaging, calcium imaging, 2-deoxy glucose autoradiography, c-fos or other immediate early gene expression and functional magnetic resonance imaging. Among these methods, intrinsic and calcium imaging methods have advantages in having spatial resolution that is sufficient for distinguishing single glomerulus. In addition, these methods also have better temporal resolution so that one can apply a series of different odorants in the same animal.
Glomeruli that locate on the dorsal side of the OB are well studied using these imaging methods in rats and mice.(65)(66)(67)(68)(69)(70) These and other imaging experiments have contributed substantially to the understanding of odor coding: First, odorants can activate more than one glomeruli. Second, specific odorants activate diverse combinations of glomeruli, suggesting that different odorants activate distinct combinations of ORs. Structurally related odorants tend to activate overlapping but distinct sets of glomeruli. Third, increased concentration of a given odorant activates more glomeruli suggesting that increased concentration of odorants activates more ORs. Fourth, the activation map in the OB seems to be mostly invariant among animals, though some variability can be observed. This is consistent with a stereotyped OR targeting map in the OB. Lastly, specific glomeruli can be identified based on their activation profiles to a set of odorants.
Mechanisms of Odorant Detection by Odorant Receptors
How does the olfactory system use ORs to detect and discriminate volatile odorants? It is believed that the olfactory system use "combinatorial codes" to discriminate odorants. In general, it is likely that a given OR has different affinities for various odorants. Conversely, a given odorant would be recognized by multiple ORs in most cases. Some odorants would never bind to a given OR. Other odorants would bind to it only at high concentrations. Yet other odorants would bind to it at low concentrations. In sum, a given odorant at a given concentration would bind to a specific combination of ORs.
To understand how odorants are detected and discriminated at the molecular level, it is essential to know which OR recognizes which odorant molecules. Moreover, because ORs are the largest family among GPCRs, putative ligand binding regions of ORs, the transmembrane domains, are extremely divergent. Since there are numerous odor ligands available, this GPCR family might represent one of the best systems to identify either motifs or specific amino acid residues involved in the recognition of ligands by GPCRs. Our understanding of molecular mechanisms underlying odorant-OR interaction would be significantly strengthened if we had a set of ORs with specific ligands for the analysis of ligand specificities of these ORs to various other structurally related odorant molecules.
After the initial identification of the OR genes in 1991, one of the foci in the field of olfaction has been the identification of the cognate ligands of ORs.(71) OSNs or cell lines derived from OSNs were first used for exogenous OR protein expression.(72)(73)(74)(75) It has been hypothesized that since OR proteins are not transported to the plasma membrane in heterologous cells,(76) OSNs must have special molecular machinery that promotes proper targeting of OR proteins to cell surface of the cilia in the OSNs, even though the nature of this machinery is unknown.(74)> In fact, the use of recordings from adenovirally infected rat OSNs first successfully matched an odorant to a cloned OR.(73)
Despite the initial success in identifying OR cognate ligand, only a few ORs have been expressed and analyzed using OSNs. Though the establishment of a heterologous expression system in cell lines is essential for conducting a large scale analysis of OR ligand specificities, exogenously-expressed ORs in cell lines pose critical problems, namely, poor protein expression, poor plasma membrane trafficking and/or poor coupling to signal transduction components in widely-used heterologous cells. Intriguingly, various researchers have since attempted the addition of OR accessory factors such as a N-terminal epitope tags, signal sequences and/or putative OR chaperones to ORs in heterologous expression systems; this effort eventually led to the efficient expression of a subset of ORs in HEK293T cells and/or Xenopus oocytes and, thus, the identification of some of their cognate ligands.(21)(40)(72)(73)(77)(78)(79)(80)(81)(82)(83)(84)(85)(86)(87)(88)(89)(90)(91)(92)(93)(94)(95)
Interindividual Differences in Olfactory Perception
Individual differences in the perception of various odors can be found in everyday life. For example, the smell of cilantro is perceived by some to be pleasing and fresh, while others find it soapy and unpleasant. Musk odors are similarly perceived as animal-like, sweet or odorless. It is known that some people cannot detect mercaptan, the sulfurous compound used to odorize liquid propane gas and warn of gas leaks. One of the most striking examples of variability in smell perception is that of the sex steroid-derived odor, androstenone (5α-androst-16-en-3-one), which is variously perceived by different individuals as offensive ("sweaty, urinous"), pleasant ("sweet, floral") or odorless.(96)(97)(98)
While culture and upbringing can provide sensory experiences that influence olfaction perception and hedonics,(99)(100) there is evidence that prior exposure is not the whole story. Observations of very young infants show they pay selective attention to different smells,(101) suggesting an innate predisposition for at least some olfactory responses. Studies on the loss of perception to certain odorants provide perhaps the best evidence for an effect of genes on variability in smell perception.
Specific anosmia is the absence of olfactory percept for a particular molecule in an individual who has otherwise intact general olfactory abilities.(102)(103)(104)(105) This is usually contrasted with general anosmia, which is the total loss of olfactory sensation. The etiology for general anosmia has been well-characterized with most of these cases attributed to neurodegenerative disorders or traumatic injuries to the brain. Interestingly, the best known instance of genetically-determined general anosmia is Kallman's syndrome where developmental failure of olfactory structures in the central nervous system leads to patients' inability to smell.(106) By inference, the cause of specific anosmia may be due to more peripheral structural alterations at the odorant receptor level.
Human Polymorphisms and Olfactory Variation
Previous work related sensory variation in taste(107)(108) and color perception(109) to genetic mutations in sensory receptors. Variation in smell perception has been traditionally ascribed to cultural differences,(99) gender and age,(110) or effects of previous experience with the odor.(111)(112) Given the behavioral evidence in the androstenone specific anosmia case, it is not impossible that genetic variation could underlie the observed variation in olfactory perception in humans. Polymorphisms in OR genes or in the OR regulatory regions that affect their expression could be a factor in the more graduated type of variability that is the hallmark of human odor perception. The scientific challenge, however, is to correlate wide-ranging patterns of sensory response, or the odor perception phenotype in this case, with the underlying OR genotype.
Androstenone is one of the most thoroughly studied examples of specific anosmia. It is known that while many adults cannot detect this odor at vapor saturation, others are exquisitely sensitive to it. Androstenone is a steroid that is also considered to be a pheromone, a chemical that modifies behavior in the same species.(113) It is found in high concentrations in the saliva of male pigs and facilitates a receptive mating stance in estrous females. It is also present in human secretions such as saliva, sweat and urine.(114)(115) Specific anosmia for androstenone has been reported at a prevalence of approximately 30% in adults.(96)(98)(116) This anosmic phenotype characterized by different profiles of androstenone sensitivities was also observed among mouse inbred strains.(117)
In addition, as reported from twin studies, androstenone anosmia may have a genetic basis: The ability of one monozygotic twin to detect androstenone is highly predictive of the same ability in the second twin,(98) pointing to the possibility that androstenone anosmia may be associated with genetic variations in genes encoding the ORs for androstenone. Indeed, using a heterologous OR expression system, it was shown that a human odorant receptor, OR7D4, is selectively activated in vitro by androstenone and the related odorous steroid androstadienone (androsta-4,16-dien-3-one).(118) Furthermore, a common variant of this receptor (OR7D4 WM) contains two non-synonymous single nucleotide polymorphisms (SNPs), resulting in two amino acid substitutions (R88W, T133M; hence 'RT') that severely impair function in vitro, correlating to the in vivo data that human subjects with RT/WM or WM/WM genotypes as a group were less sensitive to androstenone and androstadienone and found both odors less unpleasant than the RT/RT group.(118) This study demonstrates the first link between the function of a human odorant receptor in vitro and odor perception.
Another example of genotype-phenotype association was seen in the case of hyperosmia or enhanced olfactory sensitivity. Menashe et al. found a strong association signal between the single nucleotide polymorphism variants in OR11H7P and sensitivity to the odorant isovaleric acid.(119) As one of the OR segregated pseudogenes in humans, which are genes that display both functional and nonfunctional alleles in the human population, the intact allele of OR11H7P exhibited a response to isovaleric acid in a Xenopus oocyte OR expression system,(119) further strengthening the association between olfactory detection threshold phenotypes and the segregating pseudogene genotype.
Perspectives
phenotype investigations will generate genetic markers for scent preferences.
In addition to the scientific advances discussed above, the long-term aims of this line of research may yield practical applications of substantial interest and commercial potential. The U.S. market for scented personal and household products such as perfumes and laundry detergents is well over $20 billion. By matching odorants to particular receptors and understandings how we smell, two new fields intrinsically relevant to the scented product market will emerge: olfactory pharmacology and olfactory pharmacogenetics.
Olfactory pharmacology is useful for designing specific agonists and antagonists for odor modulation. This technology would allow for the precise creation or masking of any scent. For example, characterization of OR7D4 as an androstenone receptor could lead to the identification of compounds that either block or activate the receptor. Since androstenone and related steroidal chemicals are known to constitute human body odor and have been implicated in modulating mood in humans, such compounds may have direct commercial relevance. Similarly, in the agricultural market, such agonists and antagonists for steroidal compounds could also impact animal husbandry by controlling mating behaviors, since androstenone is known to function as a pheromone in pigs. Olfactory pharmacology would, therefore, have far-reaching implications for both commercial and health-related fields, enabling the creation or enhancement of pleasant odorants and the suppression of noxious odorants.
As described, variation in human olfactory perception has a genetic basis. The ability to predict olfactory phenotype based on OR genotype/haplotype constitutes the new concept of olfactory pharmacogenetics. The high-throughput OR heterologous expression system will facilitate the identification of all of the major, if not all, ORs for a given compound. Further genotype-phenotype investigations will generate genetic markers for scent preferences.
In the world of consumer products, a phenotype is the equivalent of a market segment. A well-characterized market segment, for example, people who love musk but dislike the smell of roses, will allow manufacturers to target their product development and marketing efforts to these specific populations. A genetic polymorphism linked to the phenotype holds the further promise of a biological marker for these subpopulations.
At present, unfortunately, perfumes and household fragrances are formulated without regard to interindividual differences in smell perception. However, olfactory pharmacogenetics could be exploited in the long-term to formulate scents with a predictable percept by humans of different genotypes. Segmentation of the scented products market (i.e., customized scents by genotype) could lead to a worldwide renewal and expansion of the fragrance industry by increasing the numbers of people who find perfumes pleasant.
Another area of application would be in genetic testing for specific anosmias to identify people at risk for deficits in smelling dangerous compounds such as the scent of a fire or a leaking gas stove. A genetic test would be easier to administer than a battery of olfactory psychophysics tests and would serve as an early warning and detection system for specific anosmics so that monitoring devices can be installed in their homes.
Undeniably, in this post-genomic era, as the sequencing of individual human genome is becoming more and more affordable, the unified aim of the olfaction researchers and fragrance marketers is to be able to decipher the human "olfactome" for the ultimate genetic profiling of one's olfactory sense and sensibility.