Course Authors
Yi Li, B.Sc., and Herb E. Schellhorn, Ph.D.
Y. Li is a graduate student and Dr. Schellhorn is Professor, Department of Biology, McMaster University, Hamilton, Ontario, Canada.
Within the past 12 months, Y. Li and H.E. Schellhorn report no conflicts of interest or competing interests.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the genetic cause for vitamin C deficiency in humans and why dietary vitamin C intake levels should be determined on an individual basis
Discuss the reason why the loss of vitamin C producing-ability might have been beneficial in human evolution
Identify individuals for whom vitamin C supplementation may pose health risk
Discuss why vitamin C, especially when taken large quantities, is poorly absorbed and stored by the body
Describe the relationship between the effective serum concentrations of vitamin C and the therapeutic values of this vitamin in cancer and cardiovascular disease.
Vitamin C is essential for survival. Lack of vitamin C, or vitamin C deficiency, can cause scurvy, which is manifest by well-defined symptoms, including fatigue, poor wound healing, tissue degeneration and, ultimately, death. For centuries, vitamin C-containing diets were used to prevent, and sometimes to cure, scurvy, even though the nature of the nutritional component responsible was not known.
Since its discovery as a cure for scurvy in 1930s, the therapeutic applications of vitamin C, both potential and realized, have been broadened. While well accepted as a preventive agent of nutritional diseases such as scurvy, its role in advanced therapy of multifactorial diseases (e.g., cancer and heart disease) is less well established and its potential role in immunity has not yet been unequivocally demonstrated.
To fully appreciate the impact of the complex relationship of this simple molecule on human physiology and the possible therapeutic values it may confer, we must understand some of the basic properties of vitamin C. In this Cyberounds®, we seek to address the important aspects and discuss unique properties of this vitamin, and explain how these properties potentiate the clinical applications of vitamin C (Table 1).

Table 1. Review Topics.
| Table Headline |
|---|
|
Adapted from JAMA, 1999, 281:1415-1423. Copyright © 1999, American Medical Association. All Rights reserved.

What Is Vitamin C?
Scurvy is one of the greatest mysteries in the history of medicine. A disease of epidemic proportion, it has attracted the attention of both physicians and scientists. Although scurvy, as a nutritional disease, had existed for many centuries, it was not systematically characterized until extended sea voyage became common in the 16th century. This created a unique and unprecedented condition in which men were deprived of fresh fruits and vegetables for durations long enough for scurvy to manifest itself to its full extent, often resulting in death. During the period of European colonial expansion, finding a cure for, or merely a way to prevent, scurvy became not only a medical interest but also a military and economic imperative for any nation with global ambitions.
Despite its importance and the attention devoted to this endeavor, finding a cure for scurvy proved to be a difficult task because of an inadequate understanding of the disease and the lack of rigorous scientific methodologies by which hypotheses could be tested with controlled trials. Indeed, the path leading to a complete understanding of scurvy paralleled the evolution of medical sciences, from diagnoses based on assumptive beliefs rooted in mythological views of the human body to modern objective research based on empirical evidence and cause-and-effect relationship. At the end of this long and winding road, what was discovered was a simple molecule that holds not only the curative power for this disease but also other potential therapeutic effects which, to date, are still topics of immense interest.
The term "vitamin" was derived from "vital amine," a nomenclature initially used by Casimir Funk in reference to a class of micronutrients that function in prevention of scurvy. It is now clear that these compounds do not have any amino groups. However, like many other well-known misnomers, the term "vitamin" has gained such popularity that any corrections would seem impractical.
Vitamin C is a generic term used to describe all compounds that exhibit the biochemical activity of ascorbate, including ascorbic acid, dehydroascorbic acid (DHA) and ascorbate salts. Because of its effect against a broad array of diseases, vitamin C has been described over the ages as possessing magical properties by numerous admirers, from ancient naval physicians to contemporary prominent biochemists. Although its application in therapies, such as treatments of cancer and heart disease, has been the subject of controversy for many years, the importance of vitamin C in human health is universally recognized. As some have rightly put it "Nothing emphasizes the importance of vitamin C to human beings more than the effect of being without it for a relatively short time."(1)
Physical Properties
L-ascorbic acid, a naturally-occurring and biologically active form of vitamin C, exists as white crystals with a molecular weight of 176.1. It is freely soluble in water and ethanol. L-ascorbic acid has two unique enolic hydrogen atoms that give rise to two pKa values (pKa1 = 4.17 and pKa2 = 11.57, Figure 1). Although its stability varies with specific formulation, ascorbic acid solution is generally oxidized rapidly in air and alkaline media, and is sensitive to heat.

Figure 1. Structure of Ascorbic Acid.
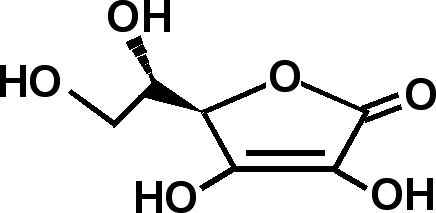

At physiological pH, L-ascorbic acid is readily deprotonated to form ascorbate, which can donate the second proton to form the resonance-stabilized ascorbyl radical, an intermediate species between ascorbate and DHA. Possessing antioxidant activity, L-ascorbic acid, L-ascorbate and DHA are collectively called vitamin C.
Biosynthesis and Evolution
Ascorbic acid is synthesized from glucose in a series of reactions. The last reaction of its biosynthetic pathway is catalyzed by an enzyme called gulonolactone oxidase (GULO). Unlike other mammals, humans carry an inactive form of the GULO encoding gene (Gulo) and, therefore, are unable to produce vitamin C and rely instead on dietary intake for survival.
This nutritional defect is caused by mutational inactivation of Gulo, which is estimated to have occurred around 40 million years ago, rendering all descending species of primate vitamin C deficient.(2) The mutated form of the human Gulo gene has, since the first inactivating mutation, acquired large deletions of several exons (Figure 2) and its remaining form is called a pseudogene.

Figure 2. Comparison Between Wild Type Mouse Gulo Sequence and Human Gulo Sequence.

Only four structurally intact exons are present in human Gulo as compared to the twelve exons that constitute a functional Gulo gene. The remaining human Gulo exons are located to the region encoding the C-terminal domains of GULO.

This mutation had been maintained and spread within the primate population and raises the question as to whether the consequent nutritional defect had conferred certain selective advantages in evolution. While the answer is unknown, a number of hypotheses regarding this loss have been proposed.(3)(4)(5) For example, some speculated that this genetic defect was initially caused by random oxidation of DNA by free radicals and followed by a virus attack that specifically targeted Gulo exons.(3) The nutritional consequences of the resultant defect must have been minimal at the time given the abundance of vitamin C-rich foods. However, this inability likely caused accumulation of unquenched free radicals which, in turn, resulted in an increase in mutation rate and helped propel the evolutionary transition from Anthropoidea to modern day Homo sapiens (Figure 3).(3)

Figure 3. The Inability to Produce Vitamin C Increased Mutation Rate and Accelerated the Evolution of Primates.
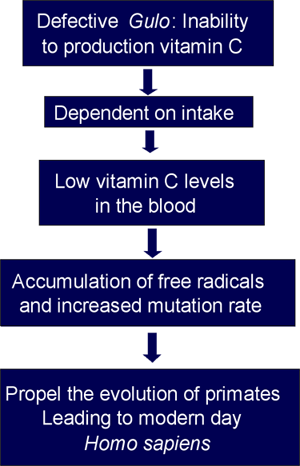
The loss of endogenous ascorbic acid reduces serum vitamin C concentrations, leaving ROS unquenched. This leads to elevated mutation rate and propels the evolution of anthropoidea and H. sapiens.
Adapted from JAMA, 1999, 281:1415-1423. Copyright © 1999, American Medical Association. All Rights reserved.

This idea of ascorbic acid influencing the rate of mutation and, consequently, evolution is supported by the observation that increased levels of free radicals promote human immunodeficiency virus (HIV) replication, whereas ascorbic acid is able to repress the replication cycle.(3) Others have proposed that the GULO-catalyzed synthesis of vitamin C is metabolically expensive and often leads to the generation of harmful by-products such as hydrogen peroxide.(6) Therefore, natural selection had favored the inactivation of this pathway, especially when the end product vitamin C is largely available from diet. The loss of vitamin C producing-ability, by reducing longevity, may have selected against aging populations, because it enhanced the availability of foods for younger and fertile individuals within populations (Figure 4).(5)

Figure 4. The Loss of Endogenous Ascorbic Acid Functions as a Selective Means to Reduce the Median Age of a Population.
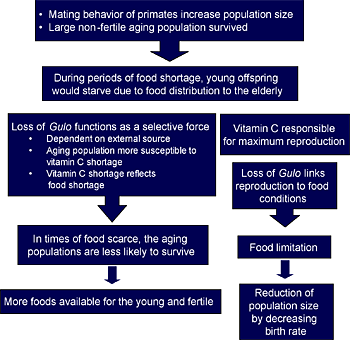
Because of their greater demand on external vitamin C, non-fertile aging populations were counter-selected during food shortage, leaving more foods for the young and fertile populations.

Physiological Functions of Vitamin C and Associated Pathologies
The healing power of vitamin C had been realized long before its discovery. The idea of using lemon juice to help prevent and treat scurvy during sea voyage was conceived as early as 17th century, more than three hundred years before the chemistry of ascorbic acid was known. As is the case for many other vital biological substances, our knowledge of this vitamin was acquired by studying the pathologies associated with dietary deficiency.
The first recognized and the most historically significant vitamin C deficiency syndrome is scurvy, a fatal metabolic disease that had claimed the lives of countless seamen, thus gaining the name "the curse of the sea."(1) Scurvy consists of a number of seemingly unrelated symptoms caused by the malfunctions of cellular mechanisms that all involve vitamin C deprivation. It is now clear that ascorbic acid functions as a cofactor and electron donor in these biological reactions.
As a specific electron donor for enzymes catalyzing the post-translational hydroxylation of collagen, including proline hydroxylase, lysine hydroxylase and procollagen-proline 2-oxoglutarate 3-dioxygenase, vitamin C is essential for collagen biosynthesis.(7) In hypoascorbemic, or vitamin C deficient condition, the hydroxylation of collagen is insufficient and leads to collagen deficiency manifested as tooth loss, blood vessel fragility, impaired wound healing and connective tissue damage, the characteristic features of scurvy.
Depression and hypochondria are also common in individuals suffering scurvy.(8) These are caused by norepinephrine deficiency which results from the inadequate conversion of dopamine to norepinephrine in the absence of ascorbic acid as a cosubstrate.(8)
L-carnitine biosynthesis also requires action of two ascorbic acid dependent-dioxygenases, N-trimethyl-L-lysine hydroxylase and γ-butyrobetaine hydroxylase.(9) As a co-substrate for these enzymes, vitamin C is essential for the production of L-carnitine. Its deprivation often leads to carnitine disorder that impairs fatty acid metabolism and produces fatigue and lethargy.(10) Animal experiments have shown that vitamin C supplementation can induce the carnitine biosynthetic pathway in guinea pigs.(10)
Iron absorption is a vital cellular process. Impaired transport leads to iron deficiency anemia characterized by pallor, fatigue and weakness.(11) Ascorbic acid facilitates non-heme iron absorption from diet in a dose-dependent manner.(11) As an electron-donating agent, ascorbate is able to reduce ferric iron (Fe3+) to the more soluble ferrous form (Fe2+), which is readily absorbed via the small intestine.(12) Vitamin C also enhances the activity of ferric reductase which catalyzes the reduction of ferric iron.(13) Alternatively, ascorbic acid counteracts the inhibitory effects of phytic acid on non-heme iron absorption, hence indirectly promoting iron uptake by the cell.(14)
How Much Vitamin C Can The Human Body Store?
Vitamin C is transported by the glucose transporter or the sodium-vitamin C co-transporter (Table 2). In the small intestine, it is absorbed by the sodium-vitamin C co-transporter, diffuses into the surrounding capillaries and then enters the circulatory system.(15)(16)(17) While circulating in the blood, vitamin C is filtered from the glomerulus capillary bed into Bowman's capsule in the kidney. The filtered vitamin C is then reabsorbed into the blood, while passing through the proximal convoluted tubule of the kidney, and is again transported by the sodium-vitamin C co-transporter.(17) The difference between filtration and reabsorption constitutes renal excretion.(18)

Table 2. Properties of Vitamin C Transporters.
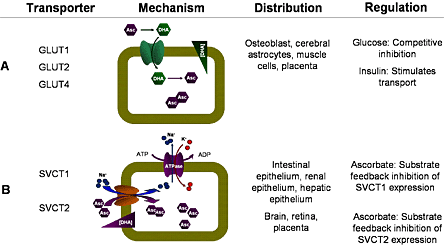

Blood levels of vitamin C are determined by both intestinal absorption and renal excretion which, in turn, reflect the activity of the sodium-vitamin C co-transporter. At low levels, orally-administered ascorbic acid is efficiently absorbed in the intestine and reabsorbed in the kidney. In contrast, in the presence of high concentrations of vitamin C, the sodium-vitamin C co-transporter becomes saturated, which, in combination with ascorbate-induced SVCT transport downregulation, attenuates both vitamin C absorption and reabsorption.(19) This attenuation gives rise to a physiological restriction on the maximal blood vitamin C concentration attainable by oral consumption, calculated to be about 200 μmol/L,(20) although normal physiological concentrations in healthy humans range from 60 to 100 μmol/L depending on levels of consumption.
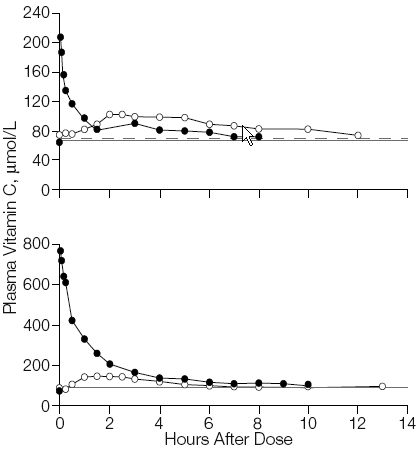
Rapid decline in serum vitamin C levels occurs within the first 2 hours of intake and results in steady-state blood concentrations of about 60 to 100 μmol/L within 6 hours(21) (Figure 5). This decline is the result of rapid renal excretion and occurs only when intake exceeds 100 mg per day.(12) Therefore, oral ingestion of large quantities of vitamin C is insufficient to raise and maintain high concentrations of ascorbic acid in the blood.

Figure 5. Reduction in Plasma Vitamin C Concentrations Over Time.

How Much Vitamin C Should One Take? Recommended Daily Allowance
The estimated average requirement (the amount of nutrient needed to meet the requirements of 1/2 of individuals within a population) have led to a proposed recommended daily allowance (RDA) of 120mg.(12) However, the current RDA for vitamin C is 75 mg for adult women and 90 mg for adult men.(90) This value is derived from the estimated average requirement (EAR) for vitamin C, which is the amount of vitamin C required by half of the healthy population at a certain life stage. This calculation takes into account several key dietary and physiological factors including dietary availability, effective serum concentration as a function of dose, bioavailability and potential adverse effects.(12)
Another RDA based on the adequate intake (AI) value has also been proposed. AI value is calculated from a group of healthy individuals and is more suitable for determining nutrient intake of individuals.(12) Based on this value, it is recommended that 200 mg of vitamin C should be taken per day, which can be acquired from 5 servings of fruits and vegetables. At this level, tissue vitamin C saturation is reached.(12) Although vitamin C can be consumed from a variety of sources, including diet and dietary supplements, it is generally recommended that vitamin C intake come directly from dietary sources. Therefore, 5 servings of fruits and vegetables, which provide 200 mg vitamin C, are recommended to healthy individuals under normal conditions.(12)
Active and Passive Smokers
The requirement for vitamin C in individuals exposed to cigarette smoking is likely higher than for the nonsmoking population. Cigarette smoke contains free radicals which can deplete circulating vitamin C in exposed individuals.(22) The reduction in serum vitamin C levels caused by smoking can reach up to 40%.(23) Nevertheless, moderate supplementation can efficiently replete serum vitamin C in smokers.(22) Therefore, for active and passive smokers to achieve levels of circulating vitamin C similar to those of non-smokers, higher levels of dietary intake are required.(24)
Individuals with Hemochromatosis
Vitamin C prevents iron deficiency anemia by facilitating iron absorption. Some have argued that the same mechanism, if it occurs in individuals with hemochromatosis, may exacerbate the risk of iron overload.(13) For example, vitamin C reduces ferric to ferrous iron through a Fenton reaction resulting in the formation of reactive radicals which are detrimental to various cellular components.(13) Indeed, multiple cell and tissue damage have been reported in patients with iron overload when supplemented with ascorbic acid.(13)(25) Therefore, it may be advisable to reduce or avoid vitamin C supplementation in these individuals.(26) The frequency of hereditary hemochromatosis in Caucasian populations is 0.4-1%,(27) and can be identified with a simple serum ferritin test or genetic testing. Physicians are encouraged to conduct such tests before recommending vitamin C supplementation in their patients, especially Caucasians.
Individuals Prone to Kidney Stone Formation
One of the metabolic end products of ascorbic acid is oxalate,(28) which can complex with calcium ion and potentially facilitate the formation of calcium oxalate kidney stones and is, in itself, a determinant of kidney stone formation.(29) A number of studies have been conducted in healthy subjects and stone-forming patients to examine the effect of vitamin C supplementation on oxalate excretion in the urine. However, due to the difficulties in oxalate assay techniques, their findings are largely inconsistent.(30) Using improved assay methods, one study reported an increase of 61% and 41% in urinary oxalate levels after supplementation with 1 or 2 g, respectively, of ascorbic acid in calcium stone-forming patients.(30) This increase may exacerbate the crystallization of calcium oxalate and, in effect, elevate the health risk in individuals with genetic predisposition toward kidney stone formation.(30) Therefore, these individuals should be cautioned regarding the potential danger of vitamin C supplementation. While vitamin C increases urinary excretion of oxalate, a direct association between kidney stone risk and vitamin C intake has not been established and, in fact, findings from several large perspective studies contradict the practice of vitamin C restriction in kidney stone prevention.(31)(32)Toxicity
Vitamin C is essentially non-toxic which, in part, is attributable to the reduction in absorption and reabsorption when high levels of vitamin C are taken orally. Intravenous injection of ascorbic acid, which bypasses the intestinal absorption, can result in blood vitamin C levels as high as 13 mmol/L, 140-fold higher than the maximum oral levels. No adverse effects at this high level have been reported.(20)
Dietary Sources
Fresh fruits and vegetables are the principle natural sources of vitamin C. Five servings of fruits and vegetables are generally recommended for healthy individuals.(12) Some of the common dietary sources of vitamin C are listed in Table 3. It should be noted, however, that vitamin C content is also dependent on methods of food preparation and storage. For example, boiling vegetables may result in 50% to 80% loss and long-term storage can also significantly reduce vitamin C content.(12)

Table 3. Dietary Sources of Vitamin C.
| Source (Size) | Vitamin C, mg |
|---|---|
| Fruit | |
| Strawberries (1 Cup, sliced) | 95 |
| Kiwi fruit (1 medium) | 75 |
| Orange (1 medium) | 70 |
| Cantaloupe (1/4 medium) | 60 |
| Mango (1 Cup, sliced) | 45 |
| Watermelon (1 Cup) | 15 |
| Juice | |
| Orange (1 Cup) | 100 |
| Grapefruit (1 Cup) | 70 |
| Fortified Juice | |
| Grape (1 Cup) | 240 |
| Apple (1 Cup) | 100 |
| Cranberry cocktail (1 Cup) | 90 |
| Vegetables | |
| Pepper, red or green Raw (1 Cup) Cooked (1 Cup) |
130 100 |
| Broccoli, cooked (1 Cup) | 120 |
| Brussels sprouts, cooked (1 Cup) | 100 |
| Cabbage Red, raw (1 Cup) White, raw (1 Cup) |
40 20 |
| Cauliflower (1 Cup) | 50 |
| Potato, baked (1 Medium) | 25 |
Levine et al. 1999. Criteria and Recommendations for vitamin C intake JAMA. 281:1415-1423. (used with permission).

Vitamin C and Cancer
The potential anticancer effect of vitamin C is topic of recent interest and controversy. The application of vitamin C in cancer prevention and treatment was first proposed in 1949, and was supported by a number of physicians and scientists, including Cameron and Pauling, who showed that the survival rate of terminal cancer patients could be improved by high-dose, intravenous vitamin C administration.(33)(34)(35) This formed the foundation for the idea of using megadoses of ascorbic acid in cancer treatment. However, a later study at the Mayo Clinic, using randomized, placebo-controlled methodology, failed to replicate Cameron's findings,(36) and thus cast doubt on the effectiveness and reliability of megadose vitamin C therapy.
The discrepancy between these two groups may, however, be attributable, at least, in part, to the difference in vitamin C administration. In the Cameron study, vitamin C was delivered orally and intravenously, whereas in the Mayo Clinic study it was administered by oral ingestion only. As described above, maximal effective vitamin C concentration attainable by oral ingestion is restricted to approximately 200 μmol/L in the blood, which is substantially lower than that attainable by intravenous injection. Therefore, it is likely that higher effective vitamin C concentration was achieved in the Cameron study, but not in the Mayo Clinic study and this difference may have contributed to the discrepancy.
Despite the extensive research and public attention, the application of vitamin C in cancer therapy has not yet been unequivocally proven effective or feasible.(37)(38) Prospective studies often report contradictory results regarding the correlation between vitamin C consumption and cancer incidence and mortality, which, in combination with the lack of validated mechanisms of action by which vitamin C exerts its anticancer functions, undermines the feasibility of using this vitamin as a therapeutic agent in cancer prevention and treatment.
Recently, with the advent of the cell culture system and the availability of appropriate animal models, the mechanisms that may contribute to the anti-cancer properties of vitamin C are being identified. As a potent biological antioxidant, vitamin C, at physiological concentrations, reduces potentially damaging reactive oxygen species (ROS), leading to the formation of relatively inert ascorbate free radicals,(39) hence attenuating the damaging effects of these ROS on DNA, protein and lipids. Indeed, consumption of vitamin C-rich foods tends to associate with reduced level of oxidative DNA damage.(40)(41)(42)(43) Interestingly, at high concentrations (0.3-20 mmol/L) ascorbic acid displays pro-oxidant properties which manifest more profoundly in tumor cells than in normal cells and lead to selective killing of tumor cells.(44)
In addition to its direct antioxidant/prooxidant effects, vitamin C interacts with a number of signal transduction pathways that alter cellular physiology and patterns of gene expression, both of which may impact cancer progression. For example, ascorbic acid enhances the expression of MLH1 and p73 and makes potential cancerous cells more susceptible to apoptosis or programmed cell death.(45) At high concentrations, ascorbic acid inhibits the activation of NFκB, which, in consequence, induces cell cycle arrest and apoptosis and, in turn, halts tumor progression.(46)(47)(48) Vitamin C also induces cell cycle arrest, restores cell cycle checkpoints by preventing the activation of the mitosis-inducing phosphatase Cdc25C(49) and thus prevents uncontrolled cell proliferation.
Recent data indicate that vitamin C prevents the accumulation of HIF-1α by altering the oxidative state of the cell(50) and functioning as a cofactor for hydroxylation inactivation of HIF-1α.(51) HIF-1α is a transcription factor induced by oxygen deficiency, a common condition in the interior of solid tumor.(52)(53) The induction and accumulation of HIF-1α give rise to the expression of a class of genes that are instrumental in tumor growth and metastasis.(53)(54) Therefore, the inactivation of HIF-1α by vitamin C may prevent the oxygen deficiency-induced adaptation of solid tumor and, in turn, tumor growth and metastasis.
These studies together provide potential mechanisms of action by which vitamin C functions as an anti-cancer agent. In light of these new developments, an interventional study using high-dose intravenous vitamin C in the treatment of cancer in human subjects was approved by the FDA and entered phase I trial in 2007. This is the first officially-conducted interventional study examining the anticancer effect of vitamin C and its findings, which are expected in 2009, should yield definitive results that will help resolve the value of this vitamin.
Cardiovascular Disease
Since oxidative damage contributes to the onset of cardiovascular disease, ascorbic acid, a potent biological antioxidant, is thought to have ameliorating effect in heart disease.(38) Consumption of vitamin C-rich foods such as fresh fruits and vegetables is correlated with a reduced risk of cardiovascular disease.(55)(56)(57) However, as consumption of healthy diets tends to associate with other healthy lifestyles, it is unclear from these epidemiological studies whether vitamin C is the causal factor in the reduction of heart disease. Indeed, contradictory findings have been reported.(58)
As in the case of cancer, the scientific community in recent years has re-oriented its focus towards identifying possible mechanisms by which ascorbic acid interacts with heart disease-inducing processes.
The initiation of cardiovascular disease is often caused by oxidative damage via reactive oxygen (ROS) or reactive nitrogen species (RNS) produced by normal metabolic processes. Accumulation of these biological by-products has several detrimental effects on the cardiovascular system. First, they induce oxidative modification of low density lipoprotein (LDL), leading to the formation of highly bioreactive oxidized LDL (oxLDL).(59) OxLDL are taken up by macrophages and give rise to cholesterol lipid-laden cells.(60)(61) These cells display increased adhesion to blood vessels(62)(63) and, in turn, cause inflammation(64)(65) and death of endothelial cells constituting the blood vessel.(66)(67) These events lead to the development of atherosclerotic lesion and, ultimately, cardiovascular malfunction. The effects of vitamin C on these pre-atherosclerotic events are multifold. For example, ascorbic acid sequesters ROS and RNS and, in effect, reduces their bioavailability in the blood.(68) It makes LDL more resistant to oxidation(68) and thus attenuates the formation of oxLDL. Vitamin C also counteracts the oxLDL-induced inflammation(69)(71)(72) and cell death,(73)(74) preventing the formation of lesion on the blood vessel. These properties together allow vitamin C to modulate LDL-induced atherosclerosis.
Additionally, ascorbic acid protects the nitric oxide biosynthetic pathway from ROS and RNS damage(75)(76)(77)(78) and ensures adequate levels of nitric oxide in the blood. As a signal molecule, nitric oxide stimulates blood vessel relaxation and permits unhindered blood flow.(79) Therefore, its stabilization by vitamin C enhances the resiliency of blood vessels. Indeed, vitamin C administration to patients with endothelial dysfunction can improve endothelium-dependent vasodilatation in these patients.(80)(81)
The anti-atherosclerotic properties of ascorbic acid are significantly augmented when applied with vitamin E, a lipid-phase antioxidant.(82) The cooperative interactions between these two vitamins form the basis for co-antioxidant therapy in the treatment of heart disease, which is becoming increasingly accepted by the medical community.(83)(84)(85)(86)(87)
In addition to its potential therapeutic value, vitamin C may have application in biomedical engineering and tissue regeneration. For example, vitamin C induces the differentiation of embryonic stem cells into cardiac muscle cells by turning on the expression of cardiac genes, such as GATA4, α-MHC, and β-MHC.(88)(89) This newly-discovered phenomenon not only suggests a role for vitamin C in early heart development, but also open the possibility of generating mature heart tissue from embryonic stem cell culture, which can then be used for heart transplantation.(88)(89)
Conclusions
While vitamin C is essential for many physiological functions of the body, especially as a cofactor for many enzymatic reactions, its role in the clinical intervention of diseases such as cancer and heart disease has not yet been unequivocally established. Nevertheless, intensive research has been devoted to elucidate the molecular mechanisms by which vitamin C interacts with disease-related processes and this has led to discoveries of novel properties of this compound with clinical significance. For example, the ability of vitamin C to selectively target and kill cancer cells,(44) protect apoptotic signal pathways(45) and attenuate hypoxia-inducible factor and the consequent oncogenic processes(50) suggest vitamin C as a potential anti-cancer agent. Indeed, these properties of vitamin C form the mechanistic basis upon which a Phase I clinical trial is being conducted.
In addition, completely unexpected functions of vitamin C that may have important medical implications have recently been discovered. For example, ascorbic acid stimulated transformation of mouse embryonic stem (ES) cells into cardiac muscle cells which may ultimately result in the application for cardiac tissue regeneration for heart transplantation.(88)(89) While still in their early stages, the applications of this new knowledge regarding vitamin C holds great promise for the future of medicine.
Recommended Articles
Vitamin C in Advanced Therapy of Cancer and Cardiovascular Disease
- Golde,D.W. Vitamin C in cancer. Integr Cancer Ther 2, 158-159 (2003). Gonzalez,M.J. et al. Orthomolecular oncology: a mechanistic view of intravenous ascorbate's chemotherapeutic activity. P R Health Sci J 21, 39-41 (2002).
- Gonzalez,M.J. et al. Orthomolecular oncology review: ascorbic acid and cancer 25 years later. Integr Cancer Ther 4, 32-44 (2005).
- Li,Y. & Schellhorn,H.E. Can ageing-related degenerative diseases be ameliorated through administration of vitamin C at pharmacological levels? Med Hypotheses (2006).
- Li,Y. & Schellhorn,H.E. New developments and novel therapeutic perspectives for vitamin C. J. Nutr. 137, 2171-2184 (2007).
Absorption and Excretion of Vitamin C
- Levine,M., Rumsey,S.C., Daruwala,R., Park,J.B. & Wang,Y.H. Criteria and recommendations for vitamin C intake. JAMA 281, 1415-1423 (1999).
- Levine,M. et al. Vitamin C pharmacokinetics in healthy volunteers: Evidence for a recommended dietary allowance. Proc Natl Acad Sci USA 93, 3704-3709 (1996).
- Wilson,J.X. Regulation of vitamin C Transport. Annual Review of Nutrition 25, 105-125 (2005).
Books
- Bown, S.R. Scurvy : How a Surgeon, a Mariner and a Gentleman Solved the Greatest Medical Mystery of the Age of Sail. (Chichester : Summersdale Publishers Ltd., 2004.)
- Davies,M., Austin,J. & Partridge,D.A. Vitamin C: Its Chemistry and Biochemistry. (Royal Society of Chemistry, Letchworth, 1991).