Course Authors
Chirag V. Shah, M.D., M.S., and Jason D. Christie, M.D., M.S.
Dr. Shah is Instructor in Medicine and Dr. Christie is Assistant Professor of Medicine, University of Pennsylvania School of Medicine, Philadelphia, PA.
Within the past 12 months, Drs. Shah reports no commercial conflicts of interest; Dr. Christie has been a consultant to Discovery Labs and GlaxoSmithKline.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the diagnostic criteria for acute lung injury and acute respiratory distress syndrome.
Assess the underlying precipitating causes of ALI and the differential diagnosis of ALI in a critically ill patient.
Discuss the pathophysiology of the acute phase of ALI responsible for the refractory hypoxemia.
Apply the ventilatory strategy that should be used in patients with ALI.
Acute lung injury (ALI) and its more severe manifestation, the acute respiratory distress syndrome (ARDS), are among the most common causes of acute hypoxemic respiratory failure in the intensive care unit (ICU). ALI complicates many medical and surgical conditions and represents a complex pathophysiologic sequela to a variety of different pulmonary and extrapulmonary insults. Initially described in the 1960s, ARDS is characterized by refractory hypoxemia, diffuse pulmonary infiltrates and decreased lung compliance.(1) ALI and ARDS both represent an acute syndrome of lung inflammation with increased vascular permeability in response to a systemic insult and differ only by their degree of hypoxemia. Since this differentiation is arbitrary (see below), for the purposes of this presentation, we prefer to use the term "ALI" to refer to the entire spectrum of ALI and ARDS.
Definitions
From its initial description in 1967 to 1994, investigators used varying defining criteria for ALI that incorporated several unifying concepts: acuity of onset, severe hypoxemia, lung infiltrates without overt congestive heart failure, and the presence of a severe predisposing insult (Table 1).(2)(3)(4)

Table 1. Most Common Precipitant Causes of Acute Lung Injury.
Direct or Pulmonary Insults |
Indirect or Systemic Insults* |
*Indirect mechanisms of ALI result from injury first to the lung endothelium followed by alveolar epithelial injury. Inflammatory cytokines and other mediators of injury (e.g., coagulation factors, oxidant stress complement, etc.) are delivered via the pulmonary circulation. In direct insults, the site of initial lung injury is the alveolar epithelium. The distinction has not been standardized.(15)

In efforts to improve the systematic identification of patients with this syndrome for enrollment in clinical trials and to promote studies on the epidemiology of ALI, in 1994 the American-European Consensus Conference (AECC) standardized the clinical definition of ALI as: acute onset, bilateral pulmonary infiltrates on frontal chest radiograph consistent with pulmonary edema (Figure 1), absence of evidence of left atrial hypertension and poor systemic oxygenation (Table 2).(5) The last criterion is measured as the ratio of arterial oxygen (PaO2) to the fraction of inspired oxygen (FiO2), or P:F ratio, and is independent of the level of applied positive end expiratory pressure (PEEP).

Table 2. North American-European Consensus Conference Criteria for Acute Lung Injury and Acute Respiratory Distress Syndrome.(5)
|

Figure 1a. ALI in a 47-Year-Old Female.
Chest radiograph (A) and computed tomography image (B) of 47-year-old lady with acute lung injury after gram negative septic shock from ascending cholangitis. After successful decompression of the biliary tree with placement of a percutaneous cholecystostomy tube and endoscopic retrograde cholangiopancreatiography with gallstone extraction, the patient was effectively managed with "lung protective" ventilation and supportive critical care. She was successfully extubated after 12 days and discharged from the hospital 17 days later.
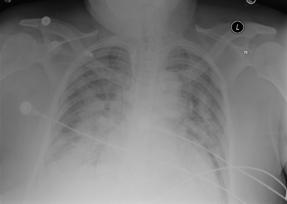
Radiograph images shows diffuse, bilateral interstitial and alveolar infiltrates

Figure 1b.
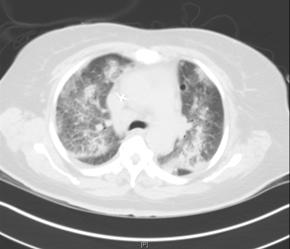
CT images shows mixed ground glass attenuation and patchy areas of consolidation. The abnormalities are diffuse and bilateral, but these heterogeneous abnormalities with areas of normal lung are better appreciated on this CT image then by the previous chest radiograph.

The syndrome is defined as ALI when the ratio is =300 mm Hg and ARDS when =200 mm Hg. Though the simplicity of the AECC criteria can be advantageous, the consensus definition has never been formally validated; it has only moderate sensitivity and specificity when compared to biopsy findings of diffuse alveolar damage, the histological proof of ALI.(6)(7)(8) In addition, there exists high inter-observer variability in the interpretation of chest radiographs among experts, wide swings in the P:F ratio after application of varying levels of PEEP and a difficulty in reliably excluding congestive heart failure both clinically or with use of a pulmonary artery catheter.(9)(10)(11)(12)(13)(14)(15) Nonetheless, the AECC definition is still widely accepted and has been successfully used in numerous epidemiological studies and clinical trials.
Epidemiology
Accurate estimates of incidence and outcomes in ALI are problematic given the wide variability in baseline characteristics of populations studied (e.g., different severity of illness, different at-risk precipitating insults, etc.), differences in methodology employed to diagnose ALI and differences in management strategies employed by investigators (e.g., ventilation, etc.). Using data from large-scale regional cohort studies, incidence estimates of ALI in the United States range from 15 to 64 cases per 100,000 person-years with an estimated 50,000 to 190,000 new cases of ALI annually in the United States.(15)(16)(17)
It is not clearly understood why certain individuals develop ALI after a predisposing insult (e.g., sepsis, trauma, etc.) and others do not. Presumably, certain individuals may have acquired or inherent predispositions for ALI, while others may have acquired or inherent protective factors for ALI.(15) The nature of these potential factors is poorly elucidated and the subject of ongoing research currently. Nonetheless, prior observational studies have suggested an association between chronic alcohol use, lack of diabetes, increasing age and transfusion of blood products with subsequent development of ALI.(18)(19)(20)(21)(22)(23)
Extrapolation of data from a recent epidemiological study suggests that 74,500 deaths may be attributable to ALI annually if one assumes a short-term mortality of ~25 to 40% after ALI, which has been the mortality rate seen in multi-center National Institutes of Health sponsored ARDS Network (ARDSNet) trials.(17), (24)(25)(26)The mortality rate after ALI appears to be highest after a predisposing insult of severe sepsis (43%) and lowest after major trauma (11%).(24)(25)(26)(27)(28)This overall mortality figure is lower than decades ago when the mortality rates approached 50-70%.(29) The exact reason for this improvement in survival is not clear, but may be related to improved ventilator strategies, supportive care in the ICU, as well as prevention and management of non-pulmonary end-organ failures.(30)(31)
Nonetheless, individual predicted mortality rates vary considerably and are contingent on a number of factors, including the type and extent of the predisposing insult, severity of illness, extent of non-pulmonary organ failures, presence of comorbidities, age and management strategies. Interestingly, the degree of baseline hypoxemia, as measured by the P:F ratio, has not been consistently associated with clinical outcomes. The exact cause of death after ALI has not been well studied. A majority of patients die from either the initial precipitating insult or from progressive end-organ failure that may partly be a consequence of local and systemic cytokine release from the injured lung.(15)(32) Progressive respiratory failure (e.g., intractable hypoxemia) appears to account for only a small fraction of deaths (<15%).(33)
The development of ALI after critical illness, regardless of the predisposing insult, appears to negatively impact both short-term (e.g., risk of death, length of hospital stay, total hospital costs) and long-term outcomes (e.g., functional and neuropsychiatric/cognitive dysfunction among survivors).(3) (34)(35)(36)(37)(38)Paradoxically, for a syndrome that predominantly affects the lungs, long-term lung function recovers to baseline in 12 months in most survivors,(39) whereas persistent neuropsychiatric and/or neuromuscular dysfunction in survivors often delays and sometimes prevents return to work.(37)(38) In the aggregate, these long-term sequelae represent an under-recognized healthcare burden attributable to ALI and represent an important aspect of its overall morbidity.
Pathophysiology
ALI may be compartmentalized into two distinct phases: an early exudative stage marked by intense inflammation and alveolar flooding followed by a late stage of cellular proliferative and potential fibrosis. Importantly, these phases have significant temporal overlap: not all patients progress to the late phase of ALI because of early recovery or death.
The exudative phase of early ALI is characterized histologically by protein-rich interstitial and alveolar edema, capillary congestion and hemorrhage in the first 24-48 hours after injury. Neutrophil infiltration ensues in the lung interstitium and alveolar compartment as a result of proinflammatory cytokines with widespread epithelial and endothelial cellular injury from oxidant stress, protease activity, complement activation and elastases. Local surfactant production is reduced, as is dilution and inactivation of existing surfactant, leading to microatelectasis. The lung milieu is transformed into a procoagulant state because of the depletion of natural anticoagulants and overexpression of antifibrinolytic proteins leading to microvascular thrombosis.
This histology of "diffuse alveolar damage (DAD)" is the hallmark of early ALI. In the next few days, condensed fibrin and protein ("hyaline membranes") form in the alveolar spaces with widespread necrosis of Type I alveolar cells. This time course of cellular changes clinically manifests as worsening tachypnea, dsypnea, cough and hypoxemia secondary to severe abnormalities of oxygen diffusion and widespread ventilation-perfusion maldistribution. The marked hypoxemia leading to pulmonary arterial bed vasoconstriction along with obliterative microvascular thrombosis can lead to elevated pulmonary pressures and right ventricular dysfunction. Eventually, the combination of increased dead-space ventilation, decreased lung compliance, increased intra-pulmonary shunt, hypoxemia and increased work of breathing leads to acute hypoxemic respiratory failure in the patient.(15)(30)
Following the initial exudative phase, some patients begin to resolve their lung injury, whereas others, for unclear reasons, enter a fibroproliferative phase characterized by resolving alveolar edema, impaired cellular healing, neovascularization and fibrosis the so-called "late phase ALI." There is disordered proliferation of Type II alveolar cells with the increased fibroblast presence. Radiographic alveolar infiltrates often improve, as do oxygenation requirements as a consequence of decreasing edema in the alveolar spaces. However, radiographic interstitial infiltrates often persist with continued elevation in dead-space ventilation and shunt, along with poor lung compliance, leading to supranormal oxygen and ventilation needs by the patient. Mechanical ventilatory support may be needed for weeks.
Diagnosis
The approach to the patient with acute hypoxemic respiratory failure from ALI should follow a systematic algorithm as the presentation of ALI can often be mimicked by other disorders (Table 3).(15)

Table 3. Differential Diagnosis of Acute Lung Injury.(15)
| Alternative Diagnosis | Comments |
|---|---|
| Hydrostatic pulmonary edema | Diastolic or systolic heart dysfunction, evidence of volume overload. Treatment includes diuresis and/or afterload reduction. |
| Diffuse alveolar hemorrhage (DAH)* | Associated with rheumatologic diseases (e.g., lupus, vasculitits) or post-stem cell transplantation. Diagnosis confirmed by bronchoscopy with bronchoalveolar lavage (BAL). Treatment may include corticosteroids plasmapheresis and/or cyclophosphamide. |
| Acute eosinophilic pneumonia* | Prodome of cough, fever, chest pain and often >15% eosinophils on BAL fluid; peripheral blood eosinophilia often absent. Treatment includes high-dose corticosteroids. |
| Acute interstitial pneumonia (AIP) | Typically slower onset than ALI (over weeks) but can acutely present over days mimicking ALI; mortality >80%. |
| Pulmonary alveolar proteinosis (PAP) | Slower onset than ALI (over months) with a characteristic computed tomography finding of "crazy paving" pattern. Treatment is whole lung lavage. |
| Hypersensitivity pneumonitis (HP) | Typically slower onset than ALI (over weeks) but can present acutely over days mimicking ALI. Removal of offending agent (if identified) is mainstay of therapy along with corticosteroids. |
| Cryptogenic organizing pneumonia (COP) | Non-infectious syndrome often precipitated by a viral infection but can be idiopathic. Onset over weeks but can present acutely over days. Typically responds to corticosteroid therapy. |
| Leukemic infiltration* | Rapid onset with evidence of active leukemia, often with concurrent blast crisis. Treatment includes leukopheresis and/or chemotherapy. |
| Lupus pneumonitis* | Often associated with active lupus. |
*Usually meet diagnostic criteria for ALI but have different pathophysiology and management.

Though many of these disorders may fulfill the criteria for ALI, they differ pathophysiologically and often require specific targeted therapies not appropriate for ALI (e.g., corticosteroids for acute eosinophilic pneumonia, etc.). Unfortunately, differentiation is often very difficult and may require rigorous diagnostic testing (e.g., laboratory, radiographic, echocardiogram, bronchoscopy) to complement the clinical history and exam (Table 4). Importantly, no single test can diagnose ALI and frequently test results may be disparate, causing diagnostic confusion.

Table 4.
Potential Diagnostic Tests in Acute Lung Injury.
(see text for details)
| Test | Comments |
|---|---|
| Arterial blood gas | Reveals severity of hypoxemia, a diagnostic criterion for ALI. However, PaO2 can be manipulated by ventilator settings and degree of PaO2:FiO2 ratio does not seem to correlate with clinical outcomes (see text). |
| Chest radiograph | Presence of bilateral infiltrates consistent with pulmonary edema is a diagnostic criterion for ALI. Infiltrates may be patchy and asymmetric. |
| Echocardiogram | Non-invasive test that can quantify left ventricular function and valvular function to ascertain the likelihood for hydrostatic pulmonary edema |
| Right heart catheterization | Invasive hemodynamic monitoring that may help differentiate causes of pulmonary edema based on pulmonary artery occlusion pressure ("wedge"). Recent data have called into question the absolute cut-off level of 18 mm Hg used in the AECC definition (see text). Patients may have co-existent cardiogenic and non-cardiogenic pulmonary edema. |
| Fiberoptic bronchoscopy (FOB) with bronchoalveolar lavage | FOB with lavage can help exclude mimickers of ALI such as diffuse alveolar hemorrhage and eosinophilic pneumonia, which require alternative management (see Table 3). |

Arterial blood gases (ABG) are needed to quantify the degree of hypoxemia and chest radiography essential to evaluate for the presence of lung infiltrates. Recently, pulse oximetry or SpO2, an indirect measure of oxygenation, was studied as a substitute for ABG. Using data from two ARDSNet clinical trials to derive and validate SpO2:FiO2 (S:F) threshold levels that correlate with P:F ratios, investigators found that an S:F ratio of 235 predicted a P:F ratio of 200 with 85% sensitivity and specificity.(40) An S:F ratio of 315 had a sensitivity of 91% and specificity of 56% for a P:F ratio of 300. Though P:F ratio is still the standard, there appears a role for S:F ratio if ABG sampling is not available.
The use of brain natriuretic peptide (BNP) plasma levels in the evaluation of patients with acute dyspnea has become increasingly common. However, their use in critical ill patients to differentiate among causes of pulmonary edema is still unclear. BNP levels can be significantly elevated in sepsis from myocardial dysfunction and in ALI from right ventricular stretch.(41) A recent prospective cohort study evaluated the role of BNP levels in critical ill ICU patients in distinguishing between pulmonary infiltrates from ALI and congestive heart failure. A BNP cut-off level with acceptable test operating characteristics (sensitivity, specificity, predictive values) was not found.(42) We do not routinely measure BNP levels to help distinguish ALI from congestive heart failure.
Invasive monitoring with a pulmonary artery catheter (PAC), or right-heart catheter (RHC), has historic significance in ALI. Recently, however, non-invasive testing has supplanted the use of PAC to rule out congestive heart failure. Much of this change in paradigm relates to the following observations. First, the AECC definition uses a PAOP cut-off of 18 mm Hg, a level that was based on expert opinion, data from myocardial ischemia literature and resuscitation practices in the early 1990s, and not evidence-based investigations in ALI.(15) Second, a retrospective analysis of data from an ARDSNet clinical trial suggests that elevated PAOP measurements are not uncommon in patients with ALI due to transient volume loading and positive pressure ventilation. In 52% of patients who met all AECC criteria on enrollment and had a PAC, 30% of subsequent PAOP measurements exceeded 18 mm Hg; in more than 80% of patients, at least one measurement exceeded 18 mm Hg.(12) This highlights the variability of PAOP measurements in critical ill patients.
We recommend interpreting elevated PAOP measurements with caution if the clinical context and other diagnostic tests support a diagnosis of ALI. In these situations, if lung infiltrates fluctuate with PAOP measurements and infiltrates clearly resolve as the PAOP level normalizes, this would strongly support a diagnosis of congestive heart failure. If upon reducing the PAOP, lung infiltrates either persist or only slightly improve, the diagnosis of ALI is still supported. It should be noted that the diagnoses of ALI and hydrostatic pulmonary edema are not mutually exclusive. In summary, given the uncertainties of interpreting PAOP measurements in critically ill patients at risk for ALI, we do not routinely use invasive monitoring with PAC to help with the diagnosis of ALI.
Management
An essential early key component in treating patients with ALI is active identification and treatment of the precipitating cause. Ventilatory management and ICU supportive measures may be inadequate if there is a failure to diagnose and manage the underlying etiology (e.g., early institution of antibiotics for sepsis, appropriate diagnostic imaging to locate a source of sepsis, etc.).
The cornerstone of managing patients with ALI is ventilatory support. Nearly all patients with ALI require invasive mechanical ventilation via endotracheal tube. Since refractory hypoxemia as a result of physiologic shunt and ventilation-perfusion mismatch is the hallmark of ALI, achieving adequate oxygenation is a fundamental priority. We aim for a goal SpO2 of = 88% and a PaO2 = 55 mm Hg. Efforts to increase mean airway pressures, typically utilizing higher levels of PEEP, are usually employed to recruit partially fluid- filled and collapsed alveoli. PEEP may also redistribute alveolar fluid into the interstitium, allowing for more available lung units to participate in gas exchange.
In addition to adequate oxygenation, the next priority in ventilation management is avoidance of ventilator induced lung injury (VILI) with "lung protective" ventilation. The concept of "lung protective" ventilation was created from the dramatic forging of two lines of research. The first line of research consisted of basic science investigations that studied the harmful effects of high volume and mechanical forces in animal models of lung injury. High volume ventilation was shown to cause and perpetuate lung injury in animals and lead to the release of local and systemic inflammatory cytokines.(43)(44)(45) The second line of research extended these observations to patients with ALI by examining the effects of systematic changes in selected ventilatory parameters with physiologic and radiographic changes.(15), (46)
Using computed tomography, investigators have shown the radiographic heterogeneity of the lung in ALI. Specifically, areas of injured lung surround areas of normal lung. This loss of healthy alveoli results in the delivered tidal volume being distributed to far fewer aerated alveoli, increasing the risk of overdistention of the remaining healthy lung units (i.e., volutrauma). Volutrauma coupled with atelectrauma, the potential injury secondary to the cyclic opening and closing of damaged alveoli, are the main components of VILI. VILI can lead to the dissemination of inflammatory cytokines from the alveolar compartment into the pulmonary and systemic circulation, potentiating multisystem organ failure.(47)
The intersection of basic science and clinical research resulted in the two hypotheses of "lung protective ventilation" in ALI. First, end-expiratory lung volume should be minimized to limit alveolar overdistention (volutrauma) and second, sufficient PEEP should be applied in order to prevent the repetitive opening and closing of alveoli during tidal ventilation (atelectrauma). In 2000, the ARDSNet investigators confirmed these hypotheses by conducting a landmark, multi-center randomized control trial of low tidal volume ventilation with 6 ml/kg ("lung protective") vs. traditional tidal volume ventilation with 12 ml/kg.(26) Weight was calculated using the predicted body weight (PBW) equation and not based on actual body weight (Table 5).

Table 5. Predicted Body Weight (PBW) Equations for Tidal Volume Calculations in Acute Lung Injury.
See http://www.ardsnet.org/node/77460
| Gender | Calculation for PBW in kilograms |
|---|---|
| Female | 45.5 + 2.3 [height(inches) - 60] |
| Male | 50.0 + 2.3 [height(inches) - 60] |

In both groups, plateau pressures (end-inspiratory pressures) were limited to =30 mm Hg with additional tidal volume reductions if this limit was exceeded. The use of "lung protective" strategy (i.e., 6 ml/kg PBW) did result in significantly higher respiratory rates, higher PaCO2 levels, lower pH levels and lower P:F ratios. However, the trial was stopped early after interim analysis showed a significant mortality benefit (31% vs. 40%) in the 6 ml/kg arm. This trial set the standard by which ALI patients are ventilated today.
An important point to remember is that avoidance of VILI takes precedent over normalization of PaCO2 and pH levels, which is contrary to some past goals of mechanical ventilation in ALI. Lower tidal volumes are often coupled with high set respiratory rates (e.g., 30-35 breaths per minute) to prevent severe respiratory acidosis. Goal pH levels in patients receiving "lung protective" ventilation are 7.25-7.40. Intravenous sodium bicarbonate may be necessary for severe respiratory acidosis caused by low tidal volumes despite high respiratory rates ("permissive hypercapnia"). Although there is no consensus pH at which sodium bicarbonate should be administered, a pH >7.25 may be employed at clinician discretion. During assisted ventilation, judicious use of sedatives is required to synchronize the patient's respiratory efforts with the ventilator and to decrease oxygen consumption. Neuromuscular blockade may be considered in cases of severe patient-ventilator synchrony not amenable to heavy sedative use, but likely increases the risk of diffuse neuromuscular weakness in survivors.
Despite the numerous modes and strategies of mechanical ventilation, very few have been rigorously studied in randomized controlled trials other than low tidal volume ventilation with volume-assist control. Though alternative modes of ventilation can provide much higher mean inflationary pressures and improved alveolar recruitment (e.g., airway pressure release ventilation, pressure control with inverse ratio), we still recommend using the ARDSNet "lung protective" strategy as the core ventilator management strategy in ALI. This is based both on the results of the ARDSNet studies as well as also on the plethora of basic and clinical studies that support the physiological basis and efficacy of this mode of ventilation.
The ARDSNet investigators have studied several other treatment modalities in ARDS (e.g., corticosteroids, fluid management, etc.). The results of these multi-center trials are briefly summarized in Table 6.

Table 6. Summary of Select NIH ARDS Network Sponsored Randomized Clinical Trials in Acute Lung Injury.
See http://www.ardsnet.org/node/733 for details
| Trial | Intervention | Findings |
|---|---|---|
| Lower Tidal Volume Trial (ARMA)(26) | 6 ml/kg vs. 12 ml/kg tidal volume for ventilator management | 6 ml/kg tidal volume arm had 9% absolute mortality risk reduction, less organ dysfunction and shorter duration of ventilation (see text). |
| Positive End Expiratory Pressure Trial (ALVEOLI)(24) | Higher PEEP vs. Traditional levels of PEEP for ventilator management | PEEP levels in control arm were based on ARMA study. Higher PEEP group had improved oxygenation but no overall improvement in mortality. |
| Late Steroid Rescue Study (LaSRS)(25) | Use of corticosteroids to improve lung repair in "fibroproliferative" stage of ALI | No difference in short-term mortality or risk of infection between placebo and steroid arms. Steroid group had a higher rate of neuromuscular weakness. |
| Fluid and Catheter Treatment Trial (FACTT)(28)(48) | Pulmonary artery catheter (PAC) vs. central venous catheter (CVC) to guide ALI treatment Restrictive ("dry") vs. liberal ("wet") fluid management in ALI |
PAC-guided therapy did not improve survival or organ function but was associated with more complications than CVC-guided therapy. No difference in short-term mortality. The "dry" arm had improved lung function and shorter duration of mechanical ventilation. |
| Early vs. Delayed Nutrition Trial (EDEN) | Early vs. delayed full caloric feedings in management of ALI patients | Enrolling currently (NCT00609180). |
| Omega-3 Fatty Acid Trial (OMEGA) | Supplementation of enteric feeds with Omega-3 fatty acids and antioxidants to improve outcomes in ALI | Enrolling currently (NCT00609180). |
N.B. EDEN and OMEGA are part of one trial with two interventions.

Finally, though a review of adjuncts to "lung protective" ventilation for the treatment of ALI and salvage therapies for ALI is beyond the scope of this review, Table 7 briefly reviews the rationale behind selected interventions in patients who fail low tidal volume ventilation.

Table 7. Adjunct and Salvage Therapies for Acute Lung Injury.
| Therapy | Comments |
|---|---|
| Recruitment maneuvers (RM) | Application of continuous positive airway pressure of 30-40 cm H20 for 30-40 seconds to transiently increase mean airway pressures in effort to rapidly "recruit" collapsed alveoli that then can be kept "open" by high levels of PEEP. Evidence is lacking that RMs alone improve clinical outcomes in ALI. |
| Airway pressure release ventilation (APRV) | Alternative mode of ventilation that utilizes high, sustained inflationary pressures with rapid, incomplete deflationary phases. Spontaneous breathing by the patient is allowed at these high pressures to augment ventilation; may improve oxygenation endpoints but has never been studied vs. the ARDSNet "lung protective" strategy. |
| Prone positioning | In ALI, majority of lung infiltrates are in the dependent lung regions. Prone positioning may improve oxygenation by increasing functional residual capacity, changing regional diaphragmatic positioning and redistributing blood flow and ventilation to the non-dependent, least affected areas of lung. Clinical trials with prone positioning have shown improved oxygenation but no effect of mortality in ALI. |
| Inhaled prostacyclin and nitric oxide | Reduces pulmonary vascular resistance and improves oxygenation by selectively vasodilating pulmonary capillaries and arterioles serving adequately ventilated alveoli. Neither has shown a beneficial effect on mortality in ALI. |
| High-frequency oscillatory ventilation (HFOV) | Based on the hypothesis that excessive tidal volumes cause lung injury, HFOV uses very small volumes (less than anatomic dead space) at very high frequencies, relying on gas exchange to occur by convection. No evidence exists showing HFOV improves mortality. |
| Extracorporeal membrane oxygenation (ECMO) | ECMO has been widely accepted in pediatric respiratory distress syndrome. In ALI, ECMO has shown to improve oxygenation and CO2 clearance but without clinical outcome benefits. Significant risks (e.g., bleeding, infection) exist and a specialized center is needed. |

It is important to realize that other than core ventilator management, alternative modes of ventilation, adjunct therapies and salvage options in ALI should not distract healthcare professionals from applying sound, supportive critical care. A premium should be placed on adequate nutrition, aspiration precautions, nosocomial prevention practices, appropriate sedative use, daily spontaneous breathing trials when appropriate, deep venous thrombosis prophylaxis and careful vigilance for complications of critical care.
Summary
ALI complicates a variety of medical and surgical conditions, most commonly severe sepsis, pneumonia and massive trauma. The syndrome is characterized by the acute onset of bilateral pulmonary infiltrates and refractory hypoxemia in the absence of overt congestive heart failure. Aggressive efforts should be made to identify and treat the precipitating cause of ALI. The cornerstone of ventilatory management is a "lung protective" strategy with low tidal volumes (6 ml/kg PBW) with limits to end-inspiratory pressures (e.g., plateau pressure <30 mm Hg) at the expense of not fully correcting respiratory acidosis. Clinical outcomes after ALI are improving but mortality, even in large academic centers, remains 25-40%. Long-term survivors typically have recovery of respiratory function but are at high risk for neuromuscular weakness and neuropsychiatric dysfunction.