Course Authors
Kirsten Bechtel, M.D.
Dr. Bechtel is Associate Professor of Pediatrics, Yale University School of Medicine, Attending Physician in the Pediatric Emergency Department and a member of the DART Team (Child Abuse Referral and Evaluation Team) at Yale-New Haven Children's Hospital.
Within the past 12 months, Dr. Bechtel reports no conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the demographics of children with abusive and accidental head trauma
Discuss the radiation risks in children exposed to computed tomography
Discuss the use of biomarkers as a potential means to determine which children are at risk for ICI after CHT and might best benefit from computed tomography
List the clinical signs and symptoms of ICI in children after accidental closed head trauma
Describe the clinical spectrum of symptoms and signs of abusive head trauma, and its association with other injuries (retinal, skeletal)
Discuss the neurological outcomes of abusive and accidental head trauma.
Children frequently sustain head trauma. Over 400,000 children under the age of 14 years present to emergency departments with closed head trauma (CHT) each year.(1),(2) Young children under the age of four have considerable morbidity from head trauma, as this age group's prevalence of intracranial injury (ICI) is more than twice the rate of the general population and nearly twice the rate of older children.(2) Many children who are symptomatic after CHT (i.e., children with headache, vomiting or mental status changes) will frequently undergo computed tomography (CT) to exclude ICI.
Despite the presence of such posttraumatic symptoms, most children will not have ICI after CHT. However, brain injury secondary to child abuse causes considerable morbidity and mortality in children less than two years of age and is the most common cause of traumatic death in children under one year of age.(3) The practitioner faces two issues when evaluating children with head trauma. First, which patients are at risk, based on their history and physical exam, for significant injury requiring diagnostic imaging and possible surgical intervention? Second, in which children should one suspect that the head injury results from child abuse?
Diagnosis of ICI
At present, the gold standard for the diagnosis of ICI in the Emergency Department (ED) setting is cranial CT.

Figure 1. Young Infant with a Non-displaced Fracture of the Right Parietal Bone with Overlying Scalp Edema.
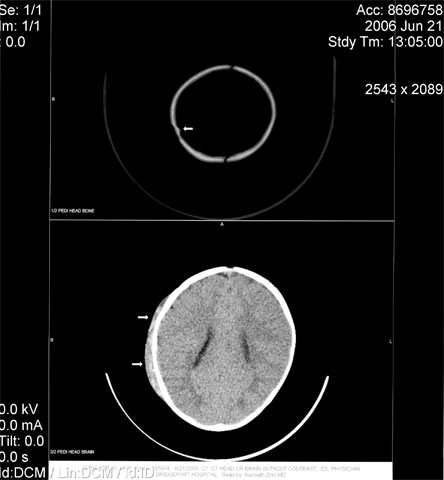

Children with CHT who undergo CT scanning sometimes require sedation to produce a more precise image of the brain. Yet both sedation and diagnostic imaging carry serious health risks for children. Adverse sedation events, defined as death and permanent neurological injury, are associated with drug overdose and drug interactions.(4) Negative outcomes are particularly frequent in hospital settings when three or more drugs are employed to sedate a patient. Furthermore, the lack of specific pediatric labeling on medication makes children vulnerable to the possibility of an adverse sedation event; both transcription mistakes and flawed dose dispensing occur in the United States with sufficient regularity to raise alarm.(4)
In addition to the risk of adverse outcome from sedation errors, children are also exposed to a significant amount of radiation when undergoing a CT scan. Although the recent development of the faster helical CT scan has reduced the need for sedation in pediatric patients, a much higher radiation dose is required.(5) Children are significantly more radiosensitive than adults, as their larger numbers of dividing cells are very susceptible to radiation-induced neoplastic transformation.(6)
More than 600,000 abdominal and cranial CT examinations are performed annually in the United States in children under the age of 15 years. Approximately 500 of these patients will eventually develop cancer attributable to the CT radiation.(7) Not only is the brain highly radiosensitive but also the risk of brain cancer, as a result of radiation exposure, increases with decreasing age. Although the development of reduced exposure pediatric CT scanners has been recommended, no such machines currently exist. Thus, the majority of symptomatic children who present to the Emergency Department with CHT undergo CT, as there is no other diagnostic method to evaluate the presence of ICI. Less than 10% of such scans in children will demonstrate ICI.(8)
Risk Factors for ICI
Several studies have tried to determine risk factors to predict which children with accidental head injury are likely to have ICI and thus benefit from cranial CT.(8),(9),(10),(11) One of the first prospective cohort studies was by Quayle (1997), who looked for predictive factors for ICI in all children presenting to the ED with head trauma.(8) Most of the children were examined with both skull radiographs and cranial CT. Predictive signs and symptoms of ICI included altered mental status, focal neurologic deficits, signs of basilar skull fracture, loss of consciousness >5 minutes and skull fracture. Headache, dizziness, vomiting, amnesia, brief loss of consciousness, scalp abrasions, contusions or hematomas were not found to be predictors of ICI.(8)
Children under two years of age were thought to be at higher risk for ICI after accidental CHT.(9),(10),(11),(12) The two initial studies to evaluate if this particular group of young children was at higher risk for ICI after CHT did not have enough data in the youngest age groups to recommend anything except a very cautious approach to evaluating head trauma in children less than two years of age.(9),(10) A later study by Greenes (1999) found an overall rate of ICI of 5% in children less than two years of age, although infants under two months of age had a prevalence of ICI of 12%.(13) Thus, it can be inferred from Greenes that diagnostic imaging should be strongly considered for children less than two who sustain accidental head trauma. However, such a practice would expose children to a substantial amount of radiation and would likely require some children to be sedated to obtain the imaging studies. Universal diagnostic imaging of very young children with closed head injury would, therefore, substantially increase resource utilization.
Because very young children are thought to be at higher risk of ICI after accidental head trauma, several investigators have chosen to specifically study children younger than two years of age with CHT to see what clinical characteristics would have a higher risk for ICI. Duhaime (1992) found that accidental head injury caused by falls from low heights is almost always benign and injuries secondary to motor vehicle accidents and falls from extreme heights were more likely to cause ICI requiring hospital admission in children younger than two years of age.(14) Shane and Fuchs (1997) reported on 102 infants <13 months old with skull fractures. Fifteen of the 102 patients were found to have ICI. The investigators demonstrated that patients who are lethargic prior to presentation or in the Emergency Department, as well as patients with parietal skull fractures, were more likely to have ICI.(15)
Greenes and Schutzman (1999) and Gruskin and Schutzman (1999) evaluated infants younger than one year of age who presented to the emergency department with accidental head trauma.(13),(16) The prevalence of ICI was 12% in the 0-2-month age group, 6% in the 3-11-month age group and 2% in infants over 12 months.(13) A significant scalp hematoma was strongly associated with ICI.(13),(16) Lethargy, irritability, full or bulging fontanel and vital signs suggestive of increased intracranial pressure were all associated with ICI, while vomiting and loss of consciousness were not.(13) Children who fell from a greater height were more likely to have complications but a number of patients had skull fractures or ICI with falls less than three feet.(16) Children without a history of neurologic symptoms and a normal scalp exam were identified as a low risk group.(13)
Finally, Greenes and Schutzman (2001) evaluated children less than two years of age who sustained accidental head trauma but had no neurologic signs or symptoms. The size of the scalp hematomas, location of scalp hematomas (parietal and temporal) and age less than three months were all associated with skull fractures. They also found that skull fracture, large scalp hematomas and parietal location were associated with ICI.(17)
Clinical Evaluation Guidelines
An expert panel used these and other studies to develop a set of guidelines for the evaluation of children <2 with minor head trauma:
- The younger the child, the lower the threshold should be for diagnostic imaging with children under 12 months and especially those under three months being at the highest risk. However, one must consider the risks of radiation exposure and possible sedation when considering cranial computed tomography in this group of children.
- The number of signs and symptoms should be considered when contemplating diagnostic imaging studies.
- Mechanism of injury including height of the fall, density of the impact surface and injury in a motor vehicle crash should be considered.
The panel of experts stratified children using clinical features including depressed mental status, focal neurologic findings, signs of basilar skull fracture, seizure, irritability, skull fracture, repeated vomiting, loss of consciousness and other characteristics into four risk categories. The categories may be useful in determining need for imaging, period of observation and disposition. It is interesting to note that the panel included seizure, vomiting and loss of consciousness despite lack of available data to support these findings as independent predictors of ICI. These proposed guidelines should be consulted for further detail.(18)
Algorithms to Identify Children at the Greatest Risk for ICI
More recent studies have tried to determine which children of all ages are at greatest risk for ICI after CHT by examining the usefulness of clinical decision rules. Oman et al. studied the sensitivity of conditions such as loss of consciousness, skull fracture, neurological deficit, persistent vomiting and abnormal behavior to determine which patients with head trauma would likely have ICI.(19) Sixteen hundred sixty-six (1,666) children with head trauma who underwent CT imaging in 21 participating Emergency Departments were examined. Of this sample, 8% (138) of patients had ICI as detected by CT. The independent use of clinical criteria such as loss of consciousness and abnormal behavior correctly identified ICI in 136 out of 138 patients.
Dunning et al. evaluated a similar clinical decision rule for the management of children with CHT in order to limit the number of patients who undergo diagnostic imaging. This prospective NEXUS II cohort study enrolled 22,772 patients younger than 16 years of age who presented to 10 emergency departments across England with CHT.(20) CT scans were ordered for children with any of the following conditions: witnessed loss of consciousness, history of amnesia, three or more episodes of vomiting, seizure, Glasgow Coma Scale Score <14, signs of basilar skull fracture, a focal neurological deficit, or if the patient history included a motor vehicle accident, high-speed injury or fall greater than 3 meters in height. Intracranial injuries of four patients were missed using this algorithm (98% sensitivity and 87% specificity).(20) Validation of this study is required, however, before emergency physicians can confidently apply this algorithm as they assess which children should undergo a CT scan for CHT.
One of the biggest limitations of such clinical decision rules is the type of wording and inter-observer reliability of interpretation of clinical predictors such as headache. A patient who describes his/her headache as 'severe' following ICI is perhaps more likely to get a CT scan than a patient who fails to further delineate the nature of his/her headache as a physician reads through a list of clinical predictors.
Sun et al. examined the difference in clinicians' interpretations of clinical predictors used to determine whether to obtain a CT scan for a pediatric patient with ICI.(21) Although some definitions such as 'abnormal mental status' and 'skull fracture' were similar between the two studies by Sun and Dunning, the definitions for clinically relevant 'vomiting' and 'headache' were different. Whereas the study by Sun defined 'vomiting' as 'history of vomiting,' the NEXUS II study defined 'vomiting' as 'inclusive of recurrent, projectile or forceful emesis after trauma, or vomiting associated with altered sensorium.'
In a similar fashion, the UC Davis definition of headache in Sun was much broader than the definition provided by Dunning utilizing NEXUS II data. Sun determined that the use of the specific NEXUS II decision instrument would have mislabeled a significant number of children with clinically relevant ICI as not being at risk for ICI.(21) Thus, clinical decision rules require carefully worded definitions and high inter-observer reliability if they are to be utilized appropriately; otherwise, they are limitations of the use of such an instrument.
Use of Serum Biomarkers to Predict ICI
Because of the complex issues that clinicians must consider in the evaluation of children for ICI after CHT, some have considered the use of serum biomarkers to see if they could identify which children are at highest risk for ICI after CHT. Over the past 10 years, researchers have examined serum biomarkers such as S100B, GFAP, IL-6 and NSE in adults to see if they could determine the presence of ICI after CHT and the extent of posttraumatic complaints in the months after head trauma. Results of these studies suggested that S100B might be more useful than other biomarkers. Specifically, S100B levels have been shown to be elevated in adults with traumatic brain injury, cerebral infarction, spontaneous subarachnoid hemorrhage, intracranial tumors, meningitis, hydrocephalus and spinal cord compression.(22),(23),(24)
In order to determine the significance of an elevated serum S100B level in patients with ICI, however, one must be able to compare it with serum S100B level in patients without brain abnormalities. Nygaard et al. attempted to define the baseline level of serum S100B in healthy adults. Blood was obtained from 110 patients between the ages of 20 and 89 undergoing surgery with spinal anesthesia.(25) Exclusion criteria included neurological disease or malignancy. The researchers employed a commercially available kit for the analysis of S100B in serum and identified the lowest detectible value as 0.2 μ/l. In this study, S100B was not detected in any patient. Therefore, Nygaard et al. concluded that S100B is normally less than 0.2 μ/l in the serum of healthy adults.(25)
Pediatric studies have examined the relationship between S100B and ICI in children with CHT. As with adult studies, it is important to determine the baseline serum S100B levels in the bloodstream of healthy infants and children. Amer-Whalin et al. noted an inverse relationship between age and serum S100B levels.(26) Infants have significantly higher normal serum S100B level than either children or adults. Maschmann et al. reported the serum S100B levels in 66 healthy neonates as 2.4-3.5 μ/l, which is significantly greater than the mean S100B level in adults (0.2 μ/l).(27) Blood was collected in 12-24 hour intervals over the first seven days of life. They concluded that amplified protein turnover in neuronal cells, combined with increased permeability of the blood brain barrier in infants, leads to the subsequent escape of S100B from the central nervous system.(27)
Spinella et al. examined the difference between serum S100B in 136 healthy children and 27 children with traumatic brain injury.(28) The mean serum S100B level in the control group of children was 0.3 µg/l and was characterized by an expected moderate inverse relationship with age. Blood was drawn within a mean of seven hours after CHT in 27 pediatric patients. A serum S100B level of 2 μ/l demonstrated 86% sensitivity and 95% specificity to predict poor outcome after six months.(28) Although a strong association between high S100B levels and poor future outcome was noted, a study with a larger number of patients is needed to determine whether S100B is indeed an independent predictor of outcome after ICI in children.
Similarly, Berger et al. measured the serum concentrations of S100B in children after mild, moderate and severe traumatic brain injury. Forty-five children between the ages of zero and 13 years with closed head injury were enrolled prospectively.(29) Blood was drawn upon arrival to the Emergency Department and every 12 hours for five days thereafter. However, the relationship of the time of injury and the time of first blood draw was not mentioned in this study. No correlation between the presence of vomiting, loss of consciousness or posttraumatic seizure and serum S100B levels was demonstrated. After 12 hours, an abnormally high S100B level was noted only in patients with severe head injury.(29) Although 49% of the patients had abnormal S100B values, 70% of the CT scans were normal. Thus, a significant number of subjects with high S100B values had normal CT scans. The researchers did not demonstrate any relationship between the level of S100B and extracranial injuries such as long bone fractures.
In order to explore the failure of CT to visualize subtle ICI, Akhtar et al. examined the relationship between high S100B levels and MRI scans in children with traumatic brain injury and negative CT scans.(30) The authors wished to determine how often both MRI and S100B were abnormal in pediatric patients with negative CT scans. Seventeen children between the ages of five and 18 years were enrolled in the study. Blood was drawn six hours and 12 hours after admission to the Emergency Department.
Akhtar et al. determined that 41% of patients with negative CT scans had identifiable lesions on MRI. There was no difference in S100B concentrations between children with positive and negative MRI, although concentrations were abnormally elevated in both cases.(30) Furthermore, S100B concentrations were higher at both time intervals in children with head and other bodily injuries compared with patients who had sustained only head injury. This result indicates that the principal extracranial sources of S100B, chondrocytes and adipocytes, likely release S100B into the bloodstream when traumatized and must be carefully considered in future studies of the usefulness of S100B in detecting ICI in children with CHT.
Abusive Head Trauma: An Entity Unique to Pediatrics
While major trauma is the most frequent cause of death in childhood, abusive head trauma (AHT) is the most common cause of traumatic death in children under one year of age.(31) Nearly 25% of children under two years of age who are hospitalized for head trauma have been abused.(31) Since the 1940s, the features of AHT, also known as "shaken baby" or "shaking-impact" syndrome, have been well established. Infants who were injured in this manner were first described in 1946.(32),(33) The first report of this entity described an infant nurse who confessed to killing three infants and injuring many more in her care. She would become angry and frustrated over an infant's crying and proceeded to grab the child about the chest or between the shoulders and elbows and shake them violently until the infant ceased to cry. Autopsies on two of these infants revealed diffuse subdural hemorrhage over both cerebral hemispheres.(32)
Pathogenesis
The biomechanical forces necessary to cause this spectrum of injuries were first described in 1972. Caffey described the "Whiplash Shaken Infant Syndrome," suggesting that the pathogenesis of subdural hemorrhage and retinal hemorrhage result from rotational deceleration forces of the head.(33) Such forces cause the dura to slide along the surface of the brain, rupturing the blood vessels in the subdural space. Similarly, the vitreous humor slides along the surface of the retina, disrupting the blood vessels that course between the layers of the retina, resulting in diffuse retinal hemorrhage.(32),(33),(34)
There has been some disagreement among those who care for children with AHT as to whether shaking alone or shaking and impact are necessary to cause this spectrum of injuries. Duhaime et al.(35) and Prange et al.(36) constructed biomechanical infant models and demonstrated that the forces necessary to cause concussion and subdural hemorrhage were generated only when these models were shaken and impacted on a fixed, hard surface; these injuries did not occur from shaking alone or during falls less than 1.5 meters. Such evidence of impact injury to the head may not be apparent on initial examination and may only be recognized when the scalp is shaved or the galea is exposed at surgery.(37)
Other investigators have suggested that vigorous, violent shaking alone generates the force necessary to cause these injuries. Gilliland and Folberg reviewed the autopsies of children who died from AHT and found that 9% did not have any evidence of focal injury to the scalp, skull or dura.(38) Similarly, Green and colleagues examined the autopsies of 16 infants who died from inflicted head trauma and found that none had evidence of impact to the head.(34) In Haviland's case series of children with AHT, only 53% of patients had evidence of contact injury (e.g., scalp bruising or skull fracture) to the head.(39) Finally, Starling evaluated the confessions of perpetrators of abusive head trauma and found that the majority admitted to only vigorous, violent shaking of victims.(40) Most of the clinical literature supports the hypothesis that some children with abusive head trauma may have sustained vigorous shaking without impact of the head on a fixed surface.
Epidemiology of AHT
Only a few studies have examined the demographic characteristics of children with AHT. Keenan et al. sought to determine the incidence of inflicted head injury of North Carolina children younger than two years of age who were admitted to a pediatric intensive care unit with traumatic brain injury over a two-year period. They found that the incidence of inflicted head injury was 17 per 100,000 during the first two years of life. The incidence was highest during the first year of life (29.7 per 100, 000) and higher for infant boys than girls (21 vs. 13 per 100,000).(41) Other factors that contributed to an increased risk of inflicted injury were young maternal age and children of multiple births.(41)
Similarly, Barlow examined the incidence and demographics of abusive head trauma in children who were admitted to pediatric hospitals with head trauma in Scotland between 1988 and 1989.(42) In this prospective study, there was an annual incidence of 24.6 per 100 000 children younger than one year. Cases were more common in urban regions, and during autumn and winter months.(42) Finally, Keenan found that the incidence of both accidental and inflicted head injury increased in areas that had been affected by a natural disaster such as a hurricane.(43) One limitation of these studies is that they only examined children who were ill enough to be admitted to a pediatric hospital; thus, both may underestimate the incidence of inflicted head injury, as they excluded children with milder injury who were not brought for medical care.
Caregivers who inflict these injuries are sometimes unaware that they have injured the child. A competent observer to such injuries, however, would inherently realize that the caregiver's actions would be injurious to the child. Infants and children are often shaken because the caregiver has unrealistic expectations of the infant or child or as a disproportionate response to increasing levels of frustration. In some instances, it may be difficult to determine if the caregiver's intent was to inflict harm or to stop the infant or child from crying. Recent studies have demonstrated that perpetrators who injure children in this manner are most likely to be, in descending order, fathers, male paramours, female babysitters and mothers.(44)
The histories provided by caregivers may be vague, "I found him like this when he awoke from a nap." There may be a suggestion of a remote, poorly defined event, "He may have fallen off the couch yesterday" or of a minor fall, "He fell and hit his head on the ground" or "He hit himself in the head with a toy." There is much literature to support the concept that household falls or falls down stairs rarely result in life-threatening brain injury, except if a space-occupying lesion such as an epidural or large intracranial hemorrhage is present.(45),(46),(47)
Signs and Symptoms of AHT
Children with AHT can have a wide spectrum of symptoms and signs. Children with milder injuries may have only irritability, vomiting, poor feeding or sleepiness. Since such symptoms overlap with common pediatric illnesses, these symptoms might not be attributed to an inflicted head injury. Children with severe inflicted head injuries often present with more ominous symptoms and signs -- apnea, unresponsiveness, seizures or cardiopulmonary arrest. The highest incidence of such injuries is in children under six months of age as a consequence of their proportionally larger head, weak neck muscles and poor head control. Varying degrees of injury can be seen in children up to two years of age.(37) Older, but physically smaller, children with developmental delays can also suffer from these injuries.
Physical Examination for Suspected AHT
On physical examination, there may not be obvious signs of trauma to the head, neck or chest. Scalp contusions may only be seen when the head is shaved or when the scalp is exposed during craniotomy.(37) The extent of brain injury largely determines the signs and symptoms a child may have. There may be neurological signs such as hypertonicity or flaccidity, gaze palsies or unequal pupils. The child may be irritable or unresponsive to pain. Focal or generalized seizures may be apparent. Some children may present with cardiopulmonary arrest from direct injury to the brainstem and upper cervical cord.(37)
Many children with abusive head trauma may have milder symptoms and signs that result in the diagnosis being overlooked. This observation was supported by a study by Jenny et al.(1999).(48) These investigators reviewed the medical records of 173 children less than three years of age with AHT. They found that 54 children (31%) had a medical evaluation shortly after sustaining inflicted head trauma but that the clinician failed to recognize that the child had been injured in this manner. Children who were most likely to have the diagnosis of AHT "missed" were less than six months old and Caucasian infants who came from families where both parents lived in the home. These infants did not have respiratory compromise or seizures, and were more likely to have only irritability or vomiting. Some of these children had nonspecific symptoms that overlapped with other common pediatric ailments, further contributing to the diagnosis being missed.(48)
Young children with cutaneous or skeletal injury from physical abuse who are without neurological signs and symptoms are also at high risk of co-existing inflicted head injury. Rubin found that of 65 children younger than two years of age who were admitted to the hospital for fractures or cutaneous injury from abuse, 37% were found to have either a skull fracture or ICI.(49) Laskey et al. reported that occult ICI was present in 29% of their sample of physically abused children younger than two years of age, and these injuries were most often seen in infants younger than one year of age.(50) Thus, one should have a low threshold for obtaining cranial imaging in young children with cutaneous or skeletal injuries from abuse despite the absence of neurological symptoms and signs.
Diagnostic Aids: Cranial CT and MRI
Cranial CT is the most rapid and reliable modality in the diagnosis of AHT. Acute subdural and subarachnoid hemorrhage, the most common brain injuries seen in children with AHT, can be readily diagnosed by CT. Typically, a subdural hemorrhage can be thin and extensive but, occasionally, it can be large enough to cause midline shift.(51)

Figure 2. Acute Subdural Hemorrhage Over the Right Temporal and Parietal Lobes in a 3-Month-Old Infant with Abusive Head Trauma.
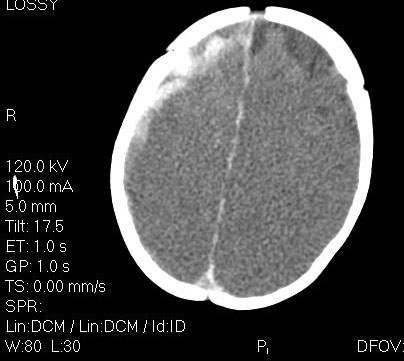

Figure 3. Acute Subdural Hemorrhage Over the Right Temporal and Parietal Lobes, in Addition to Acute Subarachnoid Hemorrhage Over the Right Tentorium in a 5-Month-Old Infant with Abusive Head Trauma.
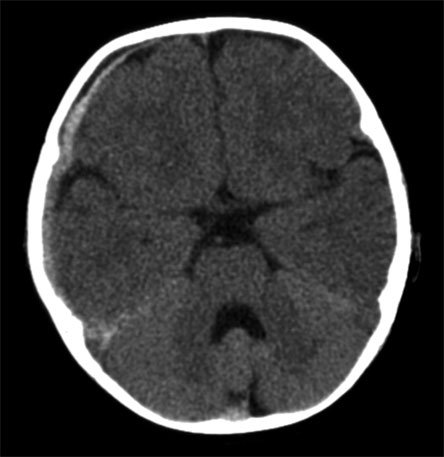
Note that this infant also has enlargement of the subarachnoid space, a benign entity found in some young infants.

Subdural hemorrhage will likely involve the interhemispheric fissure but also can involve the convexities (unilaterally or bilaterally).(6) Subarachnoid hemorrhages are usually multifocal and can be most often seen along the falx or the tentorium.(20) Skull fractures can be detected by CT scan and indicate the impact of the head onto a fixed, hard surface. Skull fractures are most often linear but can also be stellate or diastastic (significantly widened). Children with more severe deceleration injury to the brain may have evidence of diffuse cerebral edema and diffuse axonal injury. This may appear on CT as a reversal of the differentiation between the gray and white matter, also known as the "reversal sign."(31),(37)

Figure 4. Loss of Grey-White Differentiation Due to Cerebral Ddema in a 3-Month-Old Infant Who Presented with Apnea and Seizures Following an Episode of Abusive Head Trauma.
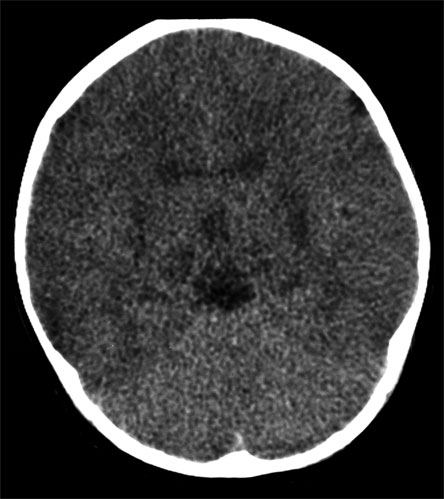

The gray matter will, therefore, appear less dense than the deeper gray matter structures of the basal ganglia and brain stem, as well as the white matter. Diffuse axonal injury arises from shearing injuries to the structures along the gray-white matter interface. This may appear as acute, punctate hemorrhage along the gray-white matter junction of the gyri, the corpus callosum or the basal ganglia.(31)
Magnetic resonance imaging (MRI) may be useful to detect small extra-axial fluid collections not appreciated on CT scans, diffuse axonal injury and to narrow the window of time in which the injury occurred.(51),(52) MRI can be difficult to obtain in children requiring mechanical ventilation and inotropic support, and should be considered as an adjunct to CT scans.
Does Your Patient Also Have Cervical Spine Injury?
The whiplash mechanism, which injures these children, raises the question of whether cervical spine injury will also be present. Postmortem examinations of children who died from abusive head trauma have demonstrated hemorrhage, contusion and axonal injury within the cervical cord.(53),(54) Premortem diagnosis of cervical cord injury, however, is unusual. Feldman et al. performed MRIs of the cervical spine in 12 children with AHT.(55) All 12 patients had a normal MRI of the cervical spine. Of these 12 patients, five died and four were found to have subdural and subarachnoid hemorrhage around the cervical cord that was not demonstrated on MRI. The extent of this hemorrhage was felt to have little clinical significance. No cervical cord injury was demonstrated at autopsy in these five patients. These authors concluded that is not productive to perform MRI of the cervical spine in infants with abusive head trauma who do not have signs of spinal cord injury.
There are few case reports of infants who survived abusive head trauma and presented with signs of spinal cord injury. Ghatan described a 24-day-old infant with abusive head trauma and minimal spontaneous movements of the extremities. While plain radiographs of the cervical spine were normal, computed tomography of the neck demonstrated disruption of the ring of the first cervical vertebrae. A subsequent MRI study showed ligamentous injury at the occipital-cervical junction, atlanto-occipital instability and cervical hematomyelia.(56) It may be that, in some patients with AHT, co-existing injury to the cervical spine contributes to the mortality in this syndrome.
Clearly, the initial presentation of hypoxic brain injury correlates with prognosis in victims of AHT. Kemp retrospectively identified 65 children less than two years of age with AHT.(57) Thirty-four percent had documented apnea at presentation that was strongly correlated with hypoxic cerebral edema and subsequent death or significant disability. When compared to a group of children with witnessed accidental head injury and subdural hemorrhage, only one had apnea at initial presentation and only two had diffuse brain edema. Similarly, Haviland et al. found that 30% of patients in their series presented with cerebral edema and raised intracranial pressure, and these patients had a dismal prognosis.(39)
Retinal Signs
Retinal hemorrhages are present in 60-95% of patients with AHT.(58),(59) They may sometimes be visualized on direct ophthalmoscopy without mydriatics but often the true extent of the hemorrhages may only be appreciated with dilated, indirect ophthalmoscopy.(60) Typically, retinal hemorrhages seen in those children with AHT are multiple and often extend to the periphery in both eyes.(58),(59) Hemorrhages can occur in multiple layers of the retina, most often in the nerve fiber and ganglion cell layers, and appear to be flame-shaped. Intraretinal and preretinal hemorrhages are more often dot, blot or boat shaped hemorrhages.(58) Retinoschesis (internal splitting of the retina), macular folding and vitreous hemorrhage are other, less commonly occurring retinal abnormalities that are often associated with abusive head trauma.(58)
Retinal hemorrhages seen in abusive head trauma are distinct from those seen in accidental head trauma. Bechtel et al. performed a prospective study of hospitalized children with inflicted and accidental head injury. Retinal hemorrhage was recognized in 10% of children with accidental head trauma and the majority of these were unilateral.(59) In contrast, 60% of those with abusive head trauma had retinal hemorrhages that extended to the periphery, and these involved the preretinal layer and covered the macula.(59) When retinal injury is suspected in cases of AHT, it is important that a dilated examination by an ophthalmologist be obtained, as significant injury may be missed by a non-specialist's examination.(60)
On occasion, retinal hemorrhage and macular folding may be seen in head injuries associated with accidental mechanisms. A case series by Christian documented three cases of accidental falls and found that retinal hemorrhages were present on the same side as the subdural hemorrhage.(61) The retinal hemorrhages noted in this case series were isolated to the posterior pole of the retina, did not cover a significant surface area of the retina, did not extend to the periphery and were not accompanied by retinal folds or detachment. Schloff found that up to 8% of children with intracranial hemorrhage from accidental causes or neurosurgical procedures may have retinal hemorrhages.(62)
With respect to macular folding, Lantz reported a case of a 14-month-old child who was struck in the head by a falling television set and subsequently died.(63) In this case report, the history of injury was corroborated by a comprehensive investigation by child protective services. While retinal hemorrhage associated with macular folding is thought to be highly suggestive of abusive injury, it is not pathognomic.
Non-traumatic Causes of Retinal Hemorrhages
There are other medical causes that may be rarely associated with retinal hemorrhages. One unusual cause is cardiopulmonary resuscitation. Odom et al. performed dilated indirect ophthalmoscopy on hospitalized children who had sustained at least one minute of closed chest compressions during cardiopulmonary resuscitation. Of the 43 patients studied, only one patient had retinal hemorrhages, which were few in number. This patient also had evidence of coagulopathy at the time of the ophthalmoscopic examination.(64)
Up to 30% of newborn infants may have retinal hemorrhage that typically resolves by six weeks of age.(65) Retinal hemorrhages may rarely be seen in children after a seizure but when seen should prompt an investigation to exclude abusive head trauma.(67),(68),(69) Hyponatremia has been associated with retinal hemorrhages following a seizure, though its role is unclear in this regard.(66),(69)
Other Signs of Shaking Injury
Other hallmarks of shaking injury include posterior and anterolateral rib fractures and metaphyseal fractures. When children present acutely with brain injury from shaking, these injuries may occasionally be detected on plain radiographs at the time on initial presentation to the Emergency Department. At the time of the acute injury, such fractures may not have overlying tenderness, edema or crepitus, and there may not be loss of function of the involved extremity. Despite the high frequency of rib fractures in abuse, clinically significant injury to the lungs and heart is uncommon.
In cases where rib fractures are not initially detected by plain radiography, they may only be apparent with skeletal scintigraphy, which can be difficult to obtain in children who have significant brain injury which requires mechanical ventilation and inotropic support. Plain radiography obtained within 10 days of initial presentation may be the only modality to demonstrate these fractures in this sicker group of patients with abusive head trauma.(45) When these fractures are present with the previously described CNS and retinal findings, this constellation of injuries is pathognomic for AHT.

Figure 5. Healing Fractures of the Left Anterolateral 3rd-6th Ribs in a Child with Physical Abuse.

A rare form of AHT can occur from direct contact injury to the head. Cutaneous injury to the ear, ipsilateral brain injury and retinal hemorrhage from blows to the head have been described in children who have been physically abused.(70),(71) This constellation of injuries has been referred to as "The Tin Ear" syndrome. Children are struck on the side of the head with an object such as the hand. Impact to the ear can cause the helix to fold onto itself and be compressed against the side of the head during the blow.(70) This results in a rim of petechiae along either the inner or outer aspect of the helix. It has been postulated that when the head is struck in this manner, it undergoes rotational acceleration which may cause the ipsilateral subdural hemorrhage, cerebral edema and retinal hemorrhage.(71)
Accidental Head Injury (HI) vs. Abusive Head Trauma
Some types of accidental closed head trauma may be confused with AHT. Children who have contact injuries to the head, either from short vertical falls or blows to the head, may sustain epidural hemorrhage, subdural hemorrhage or cerebral contusion. When there is a contact injury to the head, the point of impact causes the inner table of the skull to bend inward, compressing and injuring blood vessels in the epidural or subdural space, as well as the parenchyma of the brain itself.(72)
These focal injuries may be accompanied by a skull fracture as well. At the same time of the inbending of the skull, there is also simultaneous outbending of the skull adjacent to the site of impact.(72) This mechanism puts the outer table of the skull under tension and a skull fracture may result, either proximate to or remote from the site of impact. As the skulls of infants are somewhat more elastic, the tension on the outer table of the skull as it bends outward from an impact site may not always result in a fracture.(72) These children typically do not present with significant alterations in mental status, unless the epidural or subdural hemorrhage is large enough to cause mass effect and focal cerebral edema.(72)
In contrast to children who have head injury from shaking, the outcome of children with subdural hemorrhage secondary to impact injury is typically good. In this situation, most often the hemorrhage is not extensive and spontaneously resolves within 48 hours after injury with no neurological sequelae.(73)
Outcome After Traumatic Brain Injury
Traumatic brain injury (TBI) is a common cause of acquired disability during childhood. The severity of cognitive, behavioral and emotional dysfunction that children experience after recovery from TBI seems to be dependent on several factors: severity of the TBI, age when the TBI occurred and socioeconomic status.
While much is now known about outcome following TBI in the school-aged population, recovery in infants and young children is less documented. Anderson et al. examined cognitive and behavioral functions following TBI during early childhood.(74) They compared three groups of children who sustained mild, moderate or severe TBI injuries with a healthy control group. The groups were similar with respect to pre-injury adaptive and behavioral function, psychosocial characteristics, age and gender. Using a prospective, longitudinal design, intellectual, language and memory functions were investigated acutely post-injury and again at 12 and 30 months post-injury. Results suggested a strong association between injury severity across all neurobehavioral domains. Further, 30-month outcome was predicted by multiple factors including injury severity, socioeconomic status, pre-injury adaptive abilities and age, with pre-injury child behavior and specific lesion characteristics having surprisingly little impact.
The severity of TBI plays a role in the neurocognitive outcome for school-age children. Hawley et al. evaluated outcomes in hospitalized children with TBI.(75) Children between the ages of five and 15 years at the time of TBI were followed up to two years post-injury and were grouped as to whether they had sustained mild, moderate or severe TBI. Frequent behavioral, emotional, memory and attention problems were reported by one-third of the severe group, one-quarter of the moderate and 10-18% of the mild. Personality change since head injury (HI) was reported for 148 (28%) of the children (mild 21%, moderate 46%, severe 69%). There was a significant relationship between injury severity and neurological outcomes. Following the HI, 252 (48%) had moderate disability (mild 43%, moderate 64%, severe 69%), while 270 (51%) made a good recovery (mild 57%, moderate 36%, severe 22%). There was a significant association between social deprivation and poor outcome.
In contrast, the long-term outcome of survivors of AHT tends to be poor, dependent on the severity of symptoms at initial presentation.(76),(77),(78),(79),(80) The overall mortality from such injuries can be as high as 25%.(79) Children with inflicted head injury tend to have significantly worse neurological outcomes when compared with children with accidental traumatic brain injury. Up to 45% of survivors of inflicted injury may have mental retardation, as compared with only 5% of those with accidental injury.(76) Children who present with apnea, seizures and coma are more likely to have long-term neurological sequelae including developmental delay, seizures and static encephalopathy.(79) It is important that these children have long-term follow-up, as some survivors with milder injury may only display deficits, such as attention and memory problems, once they begin school.(80) In addition, behavioral difficulties, speech and language abnormalities may not appear until the second or third years of life, though they will persist into adolescence and adulthood.(80)
Summary and Recommendations
Children frequently present to EDs after CHT. Highly symptomatic children -- those with witnessed loss of consciousness, signs of skull fracture, neurological deficit, persistent vomiting and abnormal behavior -- would most likely benefit from cranial CT. We should have a somewhat lower threshold to obtain cranial CT for younger children with CHT. Children under two years of age with a history of head trauma and a significant scalp hematoma or alteration in mental status would also more likely benefit from cranial CT.
If the CT scan demonstrates no acute brain injury, and the family and social history raise no previous concerns of abuse or neglect, we can presume that the injury was accidental. If the CT scan of the head shows an underlying brain injury, the child should be hospitalized. If the child is younger than two years of age, then during this hospitalization, a thorough ophthalmoscopic examination and skeletal radiographic survey are recommended. Retinal hemorrhages that are multiple and extend to the periphery of the retina, are associated with retinal folding, retinal detachment or retinoschesis are highly suggestive of abusive injury.(3),(23)
If there are occult fractures or older brain injury without a reasonable history of trauma, child abuse should be suspected and a report to child protective services should then be made. If retinal hemorrhages are unilateral and few, this may be consistent with accidental head injury, particularly if there is a reasonable history of an accidental fall, and provided that there are no other occult injuries or history of familial discord or domestic violence.