The Thyroid in Pregnancy
Course Authors
Tricia Westhoff, M.D., and Eli Ipp, M.D.
Dr. Westhoff is an Endocrinology Fellow, Harbor-UCLA Medical Center. She reports no commercial conflict of interest. In the past three years, Dr. Ipp has received grant/research support from Pfizer, Inc., R.W. Johnson and Novo-Nordisk. He has served as a consultant for Novo-Nordisk, SmithKline Beecham Pharmaceutical and Hoechst Marion Roussel. Dr Ipp has also served on the Speakers' Bureau for Novo-Nordisk.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Learning Objectives
Upon completion of this Cyberounds®, you should be able to:
Discuss the physiological changes in thyroid function during pregnancy
Describe the mechanisms of hypothyroidism in pregnancy
List the effects of maternal hypothyroidism upon the fetus.
Thyroid disorders are the most common endocrine disease observed during gestation. This should not be surprising given that thyroid disease is the most common endocrine disorder of all women, with 5-10 times the prevalence in men. As with any disease that occurs in pregnancy, one has to consider not only the impact upon the mother but also potential effects upon the fetus. This Cyberounds® will provide some background on the physiology of pregnancy and its impact upon thyroid function in the mother and the fetus. It will also delve into maternal hypothyroidism. A subsequent Cyberounds® will focus on hyperthyroidism in pregnancy and post-partum thyroiditis.
Management of pregnant women with thyroid disorders requires that clinicians take both the mother and the fetus into account. Thus, the extent of placental transfer of various circulating factors in the mother, including immunoglobulins, hormones or drugs, may be responsible for induction of disease in the fetus or neonate. Examples that will be discussed in more detail in this conference and the next Cyberounds®, include IgG antibodies in autoimmune thyroid disease in the mother, or therapeutic agents for treatment of hyperthyroidism that may cross the placenta and cause hyper/hypothyroidism in the fetus or newborn. Under-treatment of hypothyroidism during pregnancy has also been suggested to influence subsequent neuropsychological function of children born to such pregnancies.
Maternal Thyroid Function in Pregnancy
The Thyroid Gland and Iodine Metabolism
Pregnancy is a complex physiological state that involves a series of hormonal and metabolic adaptations of many maternal endocrine systems. The effects upon thyroid metabolism are multiple. These include an increase in serum thyroxin-binding globulin (TBG) and thyroid hormone concentrations, an increase in renal clearance of iodine and increased production and turnover of thyroxin (T4).
In pregnancy, renal clearance of iodide increases because of an increase in glomerular filtration rate. Also, maternal iodide is further compromised because iodide and iodothyronines cross the placenta and are transferred to the fetus. As a result, serum concentrations of inorganic iodide tend to decrease, although this has not been a universal finding. Women who live where iodine intake is marginal (<50 ug per day) may have an absolute or relative iodine deficiency. This results in enlargement of the thyroid gland. Even in areas of iodine sufficiency (e.g., the United States), ultrasound studies have shown that thyroid size may increase during pregnancy. The fetus requires iodide once fetal thyroid hormone production begins in the second trimester and subsequently during the second half of gestation. Deiodination of iodothyronines within the placenta also provides iodide to the fetus. Large goitres, or thyroid nodules, are not part of the physiological adaptation to pregnancy and, therefore, should be investigated on their own merits.
Effect of Pregnancy Upon Hormone Measurements
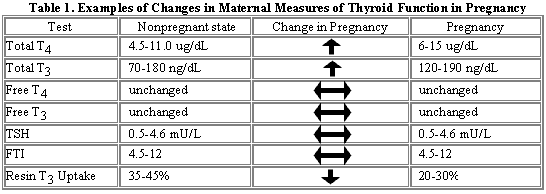
Concentrations of thyroid hormones and binding proteins change with pregnancy. TBG concentrations increase during the first trimester and plateau at 12-14 weeks gestation. Levels remain elevated throughout pregnancy at about twice as high as those seen in nonpregnant states. The increase in TBG is associated with increases in total T4 and total T3 that reflect greater binding to protein. However, levels of metabolically active forms of the hormone, free T3 and free T4 remain within the nonpregnant range.
Thus the major effect of changes in TBG is to influence laboratory tests; free hormone levels remain within normal limits. In addition, there is also a change in thyroid function that is observed in early pregnancy. An increment in free T4 concentrations occurs in the first trimester and this can be distinguished from the TBG alterations because a transient decrease in TSH concentrations occurs. The mechanism is not well understood but researchers believe it is related to placental production of human chorionic gonadotropin (HCG), which has mild thyrotropin-like action on the TSH receptor. This increase in free T4 is transient and the return to normal coincides with the decrease in HCG that occurs in the second trimester. It has been speculated that the transient increase in free T4 concentrations may be linked to the nausea that occurs in the first trimester and, possibly, even hyperemesis gravidarum.
Elevated TBG is diagnosed by direct measurement of TBG or indirectly using the Resin T3 Uptake test. As TBG increases, resin uptake decreases, reflecting increased binding capacity. The free thyroxine index (FTI) can be calculated using the Resin T3 Uptake to correct for this increase in binding by TBG. FTI = a product of total T4 and Resin T3 Uptake divided by the mean of the normal Resin T3 Uptake. This value remains relatively constant during pregnancy and can be used to follow patients with hyperthyroidism in pregnancy. Direct measurement of free hormone concentrations may also be followed in pregnant patients instead of the calculated indices. The hypothalamic-pituitary axis is not altered with pregnancy, so levels of TSH are relatively stable. A slight decrease in mean TSH concentration in the first trimester reflects the transient increase in free T4 described above that is thought to result from the effect of increasing HCG occupying the thyroid TSH receptor in 'promiscuous' fashion.
The Fetal Thyroid
Fetal thyroid development does not begin until the end of the first trimester and is not complete until delivery. Fetal thyroid follicles and T4 synthesis are first demonstrable at 10-12 weeks gestation. Development of the pituitary hypothalamic axis is also observed at 10-12 weeks gestation, as TSH is seen to increase. T4is not secreted until 18-20 weeks. Concentrations of fetal TBG and T4 continue to rise until they plateau at about 35 to 37 weeks.
When both mother and fetus are normal, the fetal thyroid develops independently of maternal influence. However, views on this have begun to change. It was thought that the placenta was totally impermeable to transfer of iodothyronines but it is understood today that limited amounts of T3 and T4 do cross from mother to fetus. Thus even fetuses with congenital absence of the thyroid gland have measurable levels of thyroid hormones in the cord blood at birth that are derived from the mother. In normal fetuses, concentrations of T3 and T4, measured in amniotic fluid or in cord blood at term, are lower on the fetal than the maternal side. TSH does not appear to cross the placenta and there is no correlation between TSH concentrations in the fetus and the mother. In contrast, inorganic iodine readily crosses and is actively concentrated by the placenta. For this reason, treatment or diagnosis with radioactive iodine is contraindicated in pregnancy for fear of damaging the developing fetal gland.
Immunoglobulins (IgG) cross the placenta. Thus in maternal Graves' disease, thyroid stimulating immunoglobulin (TSI) can theoretically cross the placental barrier and stimulate the fetal gland to become hyperthyroid. In fact, only those mothers with the most elevated TSI levels are in danger of giving birth to an infant with neonatal hyperthyroidism. Thyroid-releasing hormone (TRH) also crosses the placenta but its role in regulating fetal homeostasis is unclear. Finally, drugs used in the treatment of maternal thyroid dysfunction may cross the placenta and can potentially affect fetal thyroid function.
Monitoring fetal thyroid function is problematic. T4 can be measured in amniotic fluid but the measurements do not correlate with circulating fetal concentrations. Ultrasound can be used in the event that fetal goiter may be suspected during treatment of maternal hyperthyroidism but this is obviously a crude index of possible malfunction of fetal thyroid metabolism. Isolated information is available that describes fetal blood sampling to assess fetal thyroid function. Fetal TSH concentrations are higher, serum free T4 lower and T3 concentrations are lower than that of the mother. Soon after birth, serum TSH concentrations rapidly increase to 50-80 mU/L and then fall to 10-15 mU/L within 48 hrs. Serum T3 and T4 concentrations rapidly increase to values slightly higher than those in normal adults.
Maternal Hypothyroidism in Pregnancy
Clinical Features
Clinically obvious hypothyroidism is not a common complication of pregnancy, for many untreated women with moderate to severe hypothyroidism are infertile. In those who do become pregnant, the frequency of preeclampsia and preterm delivery are increased. A twofold increase in the rate of spontaneous abortion in women with untreated hypothyroidism has also been reported. If pregnancy occurs in a woman with clinically overt hypothyroidism, a higher incidence of stillbirth, fetal growth retardation and complications, such as anemia, preeclampsia and cardiac dysfunction, have been described.
The frequency of hypothyroidism varies among pregnant women in different countries. In surveys using TSH screening of large numbers of pregnant women in Japan, Belgium and the United States, 0.3 percent, 2.2 percent and 2.5 percent, respectively, had elevated serum TSH concentrations. Most of these women were found to have subclinical hypothyroidism, i.e., elevated serum TSH but normal serum T4 concentrations. Few were found with overt hypothyroidism (high TSH together with low T4 concentrations).
The most serious outcome of maternal hypothyroidism for the fetus is the specter of cretinism, which is most likely to occur with combined maternal and fetal hypothyroidism. This happens most often in regions with dietary iodine deficiency. The most severely affected infants have neurological cretinism, manifested by mental retardation and impaired gait and motor function. This can occur without associated hypothyroidism. Children from these areas may have normal school performance, but impaired motor and visual perceptive abilities have been reported. Iodine treatment, when provided as early as the first or second (but not third) trimester, improves neurological outcome of the child. The clinical trial that demonstrated these findings was performed in an iodine deficient area in western China.((5)
Congenital hypothyroidism, although often asymptomatic at birth, can result in severe intellectual impairment if left untreated. The incidence of this condition is 1 in every 4000 births. Sporadic cases make up 85% of thyroid dysgenesis. The others are the result of various inherited defects that cause failure of development of the thyroid gland. Because early treatment can prevent long-term consequences, screening of all newborns is performed in the industrialized world, is recommended by the American Academy of Pediatrics and is mandatory in all states. Screening is performed using an assay of neonatal serum T4.
Etiology of Hypothyroidism in Pregnancy
The most common causes of hypothyroidism in pregnancy are one or more of the following:
- Increased T4 requirements of pregnancy in women with previous thyroid disease
- Autoimmune thyroid disease
- Post-ablation therapy for Graves' disease
- Iodine deficiency.
In the United States, most women with a diagnosis of hypothyroidism in pregnancy are on inadequate T4 replacement for known hypothyroidism from Hashimoto's disease or following medically or surgically treated hyperthyroidism. Given the age group of these women, the hypothyroidism is most caused by previous Graves' disease. Obtaining this history is relevant because, even though no longer hyperthyroid, some of these women may still have thyroid stimulating immunoglobulins (TSI) that can cross the placenta and cause hyperthyroidism in the fetus (see subsequent Cyberounds®).
Hashimoto's disease is another important cause of hypothyroidism in many pregnant women. Because of the difference in prevalence of an elevated TSH in pregnancy in different countries (mentioned above), it has been suggested that there may be another possible explanation -- iodine deficiency. Iodine intake in Belgium is relatively low, only 75 to 100 µg daily, whereas in Japan it is high. In Belgium, iodine supplementation improved thyroid function and decreased the size of the thyroid in both pregnant women and their infants.
Iodine intake was thought to be sufficient in the United States but has declined in the past 15 years.(10) The median urinary iodine concentration (which correlates closely with dietary intake) was 320 ug/L from 1971 to 1974 and 145 ug/L from 1988 to 1994. During the latter period, 15% of women in the childbearing age had urinary concentrations <50 ug/L, the level of intake at which thyroid secretion is thought to be overtly inadequate. The recommended intake is 150 ug/day for adults and 200 ug/day in pregnancy. The reasons for the decline in iodide intake are not fully understood but may include a reduction in use of salt and decreased iodine supplements in bread and animal feeds. Globally, iodine deficiency affects approximately 800 million people worldwide and is thought to be the leading cause of thyroid disease affecting maternal-fetal health.
Treatment of Hypothyroidism in Pregnancy
Hypothyroidism recognized before or during pregnancy is treated with T4 replacement. The average daily dose for non-pregnant adults with hypothyroidism is approximately 100 ug but is higher in pregnancy (see below). The T4 dose is adjusted to bring TSH levels within the normal range. It takes up to six weeks for TSH values to reflect a change in dose but in pregnancy, since it is important to return (and maintain) TSH levels to the normal range as rapidly as possible (see below), levels are checked more frequently (every 3- 4 weeks) until the correct replacement dose is determined. TSH levels should then be rechecked each trimester.
Studies over the last decade demonstrate that maternal dose requirements increase in pregnancy. In 1990, Mandel(16) followed 12 women with primary hypothyroidism who were receiving exogenous T4and found that the TSH levels increased in all subjects from a mean of 2 mU/L before pregnancy to a mean of 13.5 mU/L during pregnancy. None of the women reported symptoms of hypothyroidism but because of the changes in TSH, T4 dose was increased in 9 of 12 patients. The mean dose increment was 45%. Among those patients in whom dose adjustment was not required, two had very low levels of TSH early in pregnancy, which suggested that they were being over-replaced at the start. The researchers found that increased requirements for T4 appeared in the first trimester, persisted through pregnancy and resolved postpartum. In a review of four reported series (total of 108 cases), the dose of L-thyroxine increased from a mean of 117 ug to 150 ug in the course of pregnancy.(19)
Does the increased needs for T4 in pregnancy have any clinical significance? It appears that it does. In an important recent study of the possible fetal consequences of hypothyroidism in pregnancy, Haddow et al.(9) showed that harm to the fetus might result from maternal hypothyroidism. In 62 children whose mothers had elevated TSH concentrations in the second trimester of pregnancy, differences in neuropsychological function were observed when compared to 124 mothers who were euthyroid. IQ scores at 7-9 years were slightly lower than the controls and were lowest amongst those whose mothers did not receive T4 therapy during pregnancy. The decreases in IQ scores were not large but were consistent.
While these data suggest that insufficient treatment with T4 may be responsible for the findings in the children, it must be borne in mind that other factors could also be responsible. For example, many of the mothers with hypothyroidism were anti-TPO (thyroperoxidase) positive. It has been suggested that mothers with positive antibodies and normal thyroid function may also have findings of impaired development in their children tested at 5 years of age.(18) This may have clinical significance because about 10% of women during or shortly after pregnancy may be anti-TPO antibody positive. Clearly, more studies are needed to provide information to help us understand the relationship between thyroid disease and/or its treatment and the potential impact upon the subsequent development of the children of these pregnancies.
These data would suggest that there might be a place for routine screening of TSH in pregnancy so that T4 replacement can be introduced to prevent neuropsychological consequences to the children of such pregnancies. Certainly, any patient known to have hypothyroidism should have their TSH levels evaluated during pregnancy, according to the schedule suggested above, and L-thyroxine therapy adjusted appropriately. Furthermore, a relatively inexpensive and important contribution to maternal and fetal health is to ensure that mothers are taking in adequate quantities of iodide. Not all multivitamins used in pregnancy contain adequate quantities of iodine and therefore should be checked to ensure that mothers are receiving recommended quantities.