Course Authors
Joel G. Breman, M.D., D.T.P.H.
Dr. Breman is Senior Scientific Advisor, Fogarty International Center, National Institutes of Health, Bethesda, Maryland.
Within the past 12 months, Dr. Breman reports no commercial conflicts of interests.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the global malaria burden and clinical manifestations
Diagnose and manage a patient with malaria
Discuss prevention strategies and discuss parasite resistance to existing pharmacotherapy.
Malaria is a protozoan disease with transmission in 107 countries and territories, containing close to three billion people, causing between one and three million deaths and over a billion clinical episodes each year. While malaria has been eliminated from the United States and Canada, the disease has resurged in many parts of the tropics; drug resistance of the parasite and insecticide resistance of the mosquito vectors have contributed to this resurgence. Although there are promising new control and research initiatives, malaria remains a danger to travelers going to malarious areas.
Etiology and Pahtogenesis
The four species of the genus Plasmodium that cause nearly all malarial infections in humans are P. falciparum, P. vivax, P. ovale, and P. malariae (Table 1).

Table 1. Characteristics of Plasmodium Species Infecting Humans
| P. falciparum | P. vivax | P. ovale | P. malariae | |
|---|---|---|---|---|
| Duration of intrahepatic phase (days) | 5.5 | 8 | 9 | 15 |
| Number of merozoites released per infected hepatocyte | 30,000 | 10,000 | 15,000 | 15,000 |
| Duration of erythrocytic cycle (hours) | 48 | 48 | 50 | 72 |
| Red cell preference | Younger cells but can invade cells of all ages. | Reticulocytes and cells up to two weeks old | Reticulocytes | Older Cells |
| Morphologic characteristics | Usually only ring forms; * Banana-shaped gametocytes | Irregularly shaped large rings and trophozoites; enlarged erythrocytes; Schuffner's dots | Infected erythrocyte, enlarged and oval with tufted ends; Schuffner's dots | Band or rectangular forms of trophozoites common. |
| Pigment color | Black | Yellow-brown | Dark brown | Brown-black |
| Ability to cause relapses | No | Yes | Yes | No |
* parasitemias over 2% are suggestive of P.falciparum


Figure 1. The Malaria Transmission Cycle from Mosquito to Human.
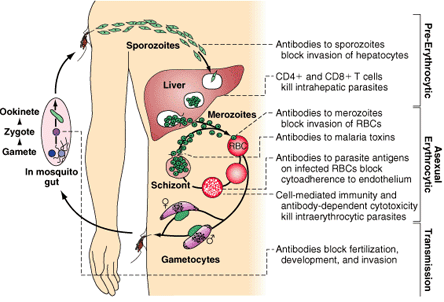
RBC = red blood cell

These motile forms are carried rapidly to the liver, where they invade hepatic parenchymal cells; the swollen infected liver cell eventually bursts, discharging motile merozoites into the bloodstream. These merozoites then invade the red blood cells (RBCs) and multiply 6- to 20-fold every 48 to 72 h. In P. vivax and P. ovale, some intrahepatic forms remain dormant for 3 weeks to a year or longer. These dormant forms, or hypnozoites, are the cause of the relapses that characterize infection with these two species.
After entry into the bloodstream, merozoites rapidly invade erythrocytes and become trophozoites; attachment is mediated via a specific erythrocyte surface receptor. In the case of P. vivax, this receptor is related to the Duffy blood-group antigen Fya or Fyb. Most West Africans and people with origins in that region carry the Duffy-negative FyFy phenotype and are, therefore, resistant to P. vivax malaria. By the end of the 48-h intraerythrocytic life cycle (72 h for P. malariae) the parasite has consumed nearly all the hemoglobin and grown to occupy most of the RBC. It is now called a schizont, Multiple nuclear divisions occur (schizogony or merogony) and the RBC then ruptures to release 6 to 30 daughter merozoites, each potentially capable of invading a new RBC.
The disease in human beings is caused by the direct effects of RBC invasion and destruction by the asexual parasite and the host's reaction. After a series of asexual cycles (P. falciparum) or immediately after release from the liver (P. vivax, P. ovale, P. malariae), some of the parasites develop into morphologically distinct, longer-lived sexual forms (gametocytes) that can transmit malaria.
After being ingested in the blood meal of a biting female anopheline mosquito, the male and female gametocytes form a zygote and then ookinete in the insect's midgut. The resulting oocyst expands by asexual division until it bursts to liberate myriad motile sporozoites, which then migrate in the hemolymph to the salivary gland of the mosquito to await inoculation into another human at the next feeding.
Epidemiology
P. falciparum predominates in Africa, New Guinea and Haiti; P. vivax is more common in Central America. The prevalence of these two species is approximately equal in South America, the Indian subcontinent, eastern Asia and Oceania (Figure 2). P. malariae is found in most endemic areas, especially throughout sub-Saharan Africa, but is much less common. P. ovale is relatively unusual outside of Africa and, where it is found, comprises <1% of isolates.

Figure 2. Malaria-Endemic Countries in the Americas (bottom) and in Africa, the Middle East, Asia, and the South Pacific (top), 2007.
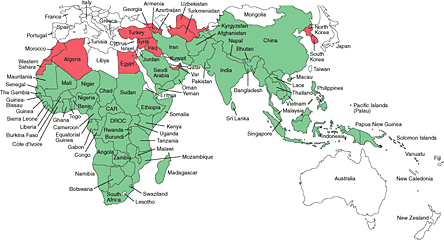
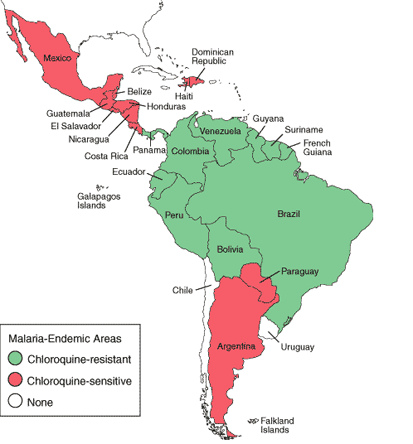

The epidemiology of malaria is dependent on Anopheles breeding sites tied to rainfall. Endemicity traditionally has been defined in terms of parasitemia rates or palpable-spleen rates in children 2 to 9 years of age as hypoendemic (<10%), mesoendemic (11 to 50%), hyperendemic (51 to 75%) and holoendemic (>75%). In holo- and hyperendemic areas' e.g., certain regions of tropical Africa or coastal New Guinea where there is intense P. falciparum transmission, people may receive more than one infectious mosquito bite per day and are infected constantly throughout their lives. In such settings, morbidity and mortality from malaria are considerable during childhood. By adulthood, however, most malarial infections are asymptomatic.
Constant year-round infection is termed stable transmission. In areas where transmission is low, seasonally limited, erratic, or focal, full protective immunity is not acquired, and symptomatic disease may occur at all ages. This situation usually exists in hypoendemic areas and is unstable transmission. Even in stable transmission areas, there is often an increased incidence of symptomatic malaria which coincides with increased mosquito breeding and transmission during the rainy season.
Malaria behaves like an epidemic disease in areas with unstable malaria, such as northern India, Sri Lanka, Afghanistan, the Sahel areas of Africa, Ethiopia, the East African highlands, Burundi, Rwanda, Madagascar, and Brazil. An epidemic can develop when there are heavy rains following drought; migrations of persons from a non-malarious region to an area of high transmission. A breakdown in malaria control and prevention services can intensify epidemic conditions, resulting in considerable mortality among all age groups. Urban and periurban malaria is increasing in importance; cities are growing rapidly in malarious areas because of migration and high birth rates.
The transmission of malaria is directly proportional to the density of the vector, the square of the number of human bites per day per mosquito, and the tenth power of the probability of the mosquito's surviving for 1 day.
ma2 pn ~ loge= vectorial capacity of vector population
innoculations of humans per infective case per day
m = anopheline density in relation to humans
a = average number of humans bitten by 1 mosquito in 1 day
p = probability of an anopheline surviving for 1 day
n = time for completion of the sporogonic cycle in the mosquito
e = base of natural logarithms, 2.71828
ma = number of bites per human per night by vector population (man biting rate)
Mosquito longevity is particularly important because the portion of the parasite's life cycle that takes place within the mosquito -- from gametocyte ingestion to subsequent inoculation (sporogony) - lasts for 8 to 30 days, depending on ambient temperature; thus, to transmit malaria, the mosquito must survive for >7 days. In general, at temperatures <16° to 18°C, sporogony is not completed and transmission does not occur.
The most effective mosquito vectors of malaria are those such as A. gambiae in Africa, which are long-lived, occur in high densities in tropical climates, breed readily and bite humans in preference to other animals. The entomologic inoculation rate -- the number of sporozoite-positive mosquito bites per person per year - is the most common measure of malaria transmission and varies from <1 in some parts of Latin America and Southeast Asia to >300 in parts of tropical Africa.
Erythrocyte Changes in Malaria
In P. falciparum infections, membrane protuberances appear on the erythrocyte's surface 12-15 hours after the red cell was invaded. These "knobs" extrude an adhesive erythrocyte membrane protein (PfEMP1) that mediates attachment to receptors on venular and capillary endothelium -- an event termed cytoadherence. This process is central to the pathogenesis of falciparum malaria, resulting in the sequestration of RBCs containing mature forms of the parasite in vital organs (particularly the brain), where they interfere with microcirculatory flow and metabolism.
Sequestered parasites continue to develop out of reach of the principal host defense mechanism. As a consequence, only the younger ring forms of the asexual parasites are seen circulating in the peripheral blood in falciparum malaria, and the level of peripheral parasitemia underestimates the true number of parasites within the body. Whereas P. vivax, P. ovale and P. malariae produce a level of parasitemia seldom >2%, P. falciparum may be associated with very high levels of parasitemia.
Host Response
Eventually, exposure to sufficient strains confers protection from high-level parasitemia and disease but not from infection. As a result of this state of infection and partial immunity without illness (premunition), asymptomatic parasitemia is common among adults and older children living in regions with stable and intense transmission (i.e., holo- or hyperendemic areas). Immunity is primarily specific for both the species and the strain of infecting malarial parasite. Both humoral immunity and cellular immunity are necessary for protection but the mechanisms of each are incompletely understood (Figure 1).
The complexity of the immune response in malaria, the sophistication of the parasites' evasion mechanisms and the lack of a good in vitro correlate with clinical immunity have all slowed progress toward an effective vaccine.
Clinical Features
The first symptoms of malaria -- lack of a sense of well being, headache, fatigue, abdominal discomfort and muscle aches followed by fever -- are all similar to the symptoms of a minor viral illness. In contrast to meningitis, there is no neck stiffness or photophobia. Myalgia is not usually as severe as in dengue fever, and the muscles are not tender as in leptospirosis or typhus. Nausea, vomiting and orthostatic hypotension are common.
The classic malarial paroxysms, in which fever spikes, chills and rigors occur at regular intervals, are relatively unusual and suggest infection with P. vivax or P. ovale. The temperature of nonimmune individuals and children often rises above 40°C accompanied by tachycardia and sometimes delirium. Although childhood febrile convulsions may occur with any of the malarias, generalized seizures are specifically associated with falciparum malaria and may herald the development of cerebral disease.
Most patients with uncomplicated infections have few abnormal physical findings other than fever, malaise, mild anemia and (variably) a palpable spleen. Anemia is common among young children living in areas with stable transmission, particularly where drug resistance is present. Splenic enlargement is found in a high proportion of otherwise healthy individuals in malaria endemic areas and reflects repeated infections. Both enlargement of the liver, particularly among young children, and mild jaundice among adults is common. Malaria is not associated with a rash like those seen in meningococcal septicemia, typhus, enteric fever, viral exanthems and drug reactions or with petechial hemorrhages seen as part of leptospirosis or viral hemorrhagic fevers.
Severe Falciparum Malaria
Promptly treated, uncomplicated falciparum malaria carries a mortality rate of ~0.1%. However, once vital organ dysfunction occurs or the total proportion of erythrocytes infected increases to >2% mortality rises steeply. The features indicating a poor prognosis are listed in Table 2.

Table 2. Features Indicating a Poor Prognosis in Severe P.falciparum Malaria.
Clinical
|
Laboratory
|
Hematology
|
Parasitology
|
PCV, packed cell volume; sGOT (AST), serum glutamic oxaloacetic transferase (aspartate aminotransferase); sGPT (ALT), serum glutamic pyruvic transaminase (alanine aminotransferase); CPK, creatine phosphokinase.

Cerebral Malaria
Coma is a characteristic feature of falciparum malaria and, despite treatment, is associated with death rates of ~20% among adults and 15% among children. The onset may be gradual or sudden following a convulsion.
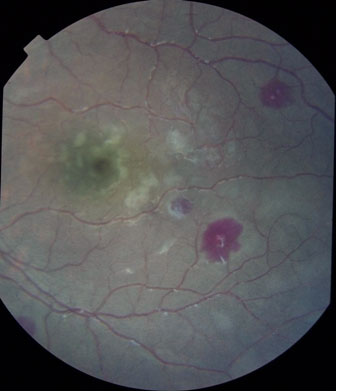
Retinal hemorrhages can be seen with pupillary dilatation and indirect ophthalmoscopy in 30 to 40% of patients. Other funduscopic abnormalities include discrete spots of retinal opacification (30 to 60%), papilledema (8% among children, rare among adults), cotton wool spots (<5%) and decolorization of a retinal vessel or segment of vessel (occasional cases) (Figure 3).

Figure 3. Thin Blood Films of Plasmodium falciparum.
A. Young trophozoites. B. Old trophozoites. C. Pigment in polymorphonuclear cells and trophozoites. D. Mature schizonts. E. Female gametocytes. F. Male gametocytes.
Reproduced from Benchaids for the Diagnosis of Malaria Infections, 2d ed, with the permission of the World Health Organization.

Convulsions, usually generalized and often repeated, occur in up to 50% of children with cerebral malaria; more covert seizure activity is also common, particularly among children. About 15% of children surviving cerebral malaria -- especially those with hypoglycemia, severe anemia, repeated seizures and deep coma -- have some residual neurologic deficit; hemiplegia, cerebral palsy, cortical blindness, deafness and impaired cognition and learning -- all of varying duration -- have been reported. There is also an increased incidence of epilepsy.
Hypoglycemia
Hypoglycemia is common in severe malaria; it is associated with a poor prognosis and is particularly problematic in children and pregnant women. Hyperinsulinemic hypoglycemia is especially troublesome in pregnant women receiving quinine treatment. In severe malaria, the usual physical signs of hypoglycemia (sweating, gooseflesh, tachycardia) are absent, and the neurologic impairment caused by hypoglycemia cannot be distinguished from that caused by malaria.
Acidosis
Acidosis is an important cause of death from severe malaria. Hyperlactatemia commonly coexists with hypoglycemia. Acidotic breathing, sometimes called respiratory distress, is a sign of poor prognosis. Lactic acidosis is caused by the combination of anaerobic glycolysis in tissues where sequestered parasites interfere with microcirculatory flow, hypovolemia, lactate production by the parasites, and a failure of hepatic and renal lactate clearance. The prognosis of severe acidosis is poor.
Noncardiogenic Pulmonary Edema
Adults with severe falciparum malaria may develop noncardiogenic pulmonary edema even after several days of antimalarial therapy. The mortality rate is >80%. This condition can be aggravated by overly vigorous administration of intravenous fluid. Noncardiogenic pulmonary edema can also develop in otherwise uncomplicated vivax malaria, where recovery is usual.
Renal Impairment
Renal impairment is common among adults with severe falciparum malaria but rare among children. This syndrome manifests as acute tubular necrosis, although renal cortical necrosis never develops. Acute renal failure may occur simultaneously with other vital organ dysfunction (in which case mortality is high) or may progress as other disease manifestations resolve.
Hematologic Abnormalities
Anemia results from accelerated RBC removal by the spleen, together with ineffective erythropoiesis. In severe malaria, both infected and uninfected RBCs show reduced deformability, which correlates with the prognosis and the development of anemia. Anemia can develop rapidly and transfusion is often required. As a consequence of repeated malarial infections, children in many areas of Africa may develop severe anemia secondary to antimalarial drug resistance.
While coagulation abnormalities and mild thrombocytopenia are common in falciparum malaria, fewer than 5% of patients with severe malaria have significant bleeding.
Liver Dysfunction
When accompanied by other vital organ dysfunction (often renal impairment), liver dysfunction carries a poor prognosis. Hepatic dysfunction contributes to hypoglycemia, lactic acidosis and impaired drug metabolism.
Other Complications
Salmonella bacteremia has been associated specifically with P. falciparum infections. HIV and malaria infections can potentiate each other. The relative incidence of complications of severe falciparum malaria is summarized in Table 3.

Table 3. Relative Incidence of Severe Falciparum Malaria Complications.
| Non-pregnant adults | Pregnant women | Children | |
|---|---|---|---|
| Anemia | + | ++ | +++ |
| Convulsions | + | + | +++ |
| Hypoglycemia | + | +++ | +++ |
| Jaundice | +++ | +++ | + |
| Renal failure | +++ | +++ | - |
| Pulmonary edema | ++ | +++ | + |
Key: - = rare
+ = infrequent
++ = frequent
+++ = very frequent

Malaria in Pregnancy
In hyper- and holoendemic areas, falciparum malaria in primi- and secundigravid women is associated with low birth weight and consequently increased infant and childhood mortality. Infected mothers in areas of stable transmission may remain asymptomatic despite intense accumulation of parasitized erythrocytes in the placental microcirculation. Maternal HIV infection predisposes pregnant women to malaria, predisposes their newborns to congenital malaria infection and exacerbates the reduction in birth weight associated with malaria.
In areas with unstable transmission of malaria, pregnant women are prone to severe infections and are particularly vulnerable to high-level parasitemia with anemia, hypoglycemia and acute pulmonary edema. Fetal distress, premature labor and stillbirth or low birth weight are common results. P. vivax malaria in pregnancy is also associated with a reduction in birth weight; this effect is more pronounced in multigravid than in primigravid women.
Malaria in Children
Most of the estimated 1 to 3 million persons who die of falciparum malaria each year are young African children. Severely anemic children may present with labored deep breathing, which in the past has been attributed incorrectly to "anemic congestive cardiac failure" but in fact is usually caused by metabolic acidosis, often compounded by hypovolemia. Evidence is accruing that severe malaria can result in long-term neurocognitive and developmental deficits.
Diagnosis
Demonstration of the Parasite
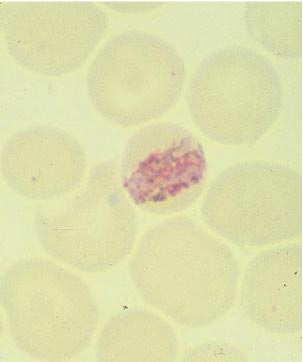
The diagnosis of malaria rests on the demonstration of asexual forms of the parasite in stained peripheral blood smears (Figures 4A, 4B, 4C).

Figure 4a. Plasmodium falciparum, Young Trophozoite: Thin Blood Film.
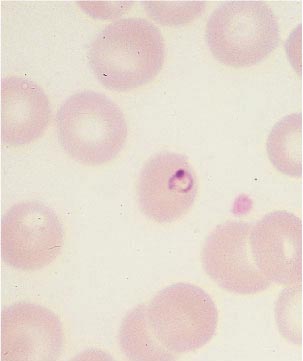
Figure 4b. Plasmodium vivax, Young Trophozoite: Thin Blood Film.
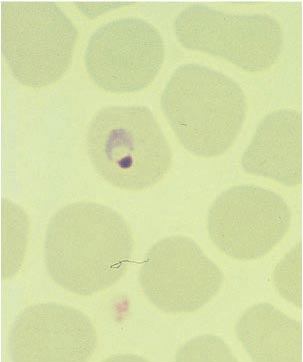
Figure 4c. Plasmodium malariae, Old Trophozoite: Thin Blood Film.

After a negative blood smear, repeat smears should be made if there is a high degree of suspicion. Of the Romanowsky stains, Giemsa at pH 7.2 is preferred; Wright's, Field's, or Leishman's stain can also be used. Both thin and thick blood smears should be examined.
The thick film has the advantage of concentrating the parasites (by 20- to 40-fold compared with a thin blood film) and thus increasing diagnostic sensitivity. Both parasites and WBCs are counted, and the number of parasites per unit volume is calculated from the total leukocyte count. Alternatively, a WBC count of 8000/μL is assumed. This figure is converted to the number of parasitized erythrocytes per microliter. A minimum of 200 WBCs should be counted under oil immersion.
Before a thick smear is judged to be negative, 100 to 200 fields should be examined under oil immersion. Rapid, simple, sensitive and specific antibody-based diagnostic stick or card tests that detect P. falciparum-specific, histidine-rich protein 2 (PfHRP2) or lactate dehydrogenase antigens in finger-prick blood samples have been introduced recently. Because the mature gametocytes of P. falciparum are not affected by most antimalarial drugs, their persistence does not constitute evidence of drug resistance.
Laboratory Findings
Normochromic, normocytic anemia is usual. The leukocyte count is normal, although it may be raised in very severe infections. The platelet count is usually reduced. Severe infections may be accompanied by prolonged prothrombin and partial thromboplastin times and by more severe thrombocytopenia. Findings in severe malaria may include metabolic acidosis with low plasma concentrations of glucose, sodium, bicarbonate, calcium, phosphate and albumin, together with elevations in lactate, blood urea nitrogen, creatinine, urate, muscle and liver enzymes, and conjugated and unconjugated bilirubin.
Treatment
Several drugs are available for oral treatment and the choice of drug depends on the likely sensitivity of the infecting parasites (Table 4). Despite recent evidence of chloroquine resistance in P. vivax (from parts of Indonesia, Oceania, East and Southern Asia and Central and South America), chloroquine remains the treatment of choice for the P. vivax, P. ovale and P. malariae except in Indonesia and Papua New Guinea, where high levels of resistance are prevalent.
Patients with severe malaria or those unable to take oral drugs should receive parenteral antimalarial therapy (Table 4). If there is any doubt about the resistance status of the infecting organism, it should be considered resistant.

Table 4. Treatment of Malaria.
| Uncomplicated Malaria | Drug treatment |
|---|---|
| Known chloroquine sensitive P. vivax, P. malariae, P. ovale, P. falciparumaa |
Chloroquine 10 mg base/kg stat followed by 5 mg/kg at 12, 24 and 36 h; or 10 mg/kg at 24 h, 5 mg/kg at 48 h or Amodiaquine 10-12 mg base/kg/day- for 3 days |
| Radical treatment of patients with P. vivax or P. ovale infections | In addition to chloroquine or amodiaquine as detailed above, primaquine 0.25 mg base/kg daily (0.375-0.5 mg base/kg in SE Asia and Oceania) should be given for 14 days to prevent relapse. In mild G6PD deficiency 0.75 mg base/kg should be given once weekly for 6 weeks. Primaquine should not be given in severe G6PD deficiency |
| Sensitive P. falciparum malariab | Artesunate 4mg/kg/day for 3 daysc + Sulfadoxine 25 mg/kg + Pyrimethamine 1.25 mg/kg (SP) single dose or Artesunate 4mg/kg/day for 3 daysc + Amodiaquine 10 mg base/kg/day for 3 daysd |
| Multidrug resistant P. falciparum malaria | Artemether-lumefantrinec 1.5/ 9 mg/kg twice daily for three days with food or Artesunatec 4mg/kg/day for 3 days + Mefloquined 25 mg base/kg (either 8mg/kg/day for 3 days or 15 mg/kg on day 2 then 10 mg/kg on day 3) |
| Second line treatments or treatment of imported malaria | Either Artesunatec 2mg/kg daily or Quinine 10 mg salt/kg three times daily for 7 days plus either a) Tetracyclinee 4 mg/kg four times daily for 7 days or b) Doxycyclinee 3 mg/kg once daily for 7 days or c) Clindamycin 10 mg/kg twice daily for 7 days or Atovaquone-Proguanil 20/8 mg/kg once daily for three days with food. |
| Severe falciparum malariaf | Artesunatec 2.4 mg/kg stat by i.v. injection followed by 2.4 mg/kg at 12, and 24 hours then daily if necessaryg, or Artemetherc 3.2 mg/kg stat. by i.m. injection followed by 1.6 mg/kg daily, or Quinine dihydrochloride 20 mg salt/kgh infused over 4 hours, followed by 10 mg salt/kg infused over 2-8 hours, every 8 hoursi or Quinidine 10 mg base/kgh infused over 1-2 hours followed by 1.2 mg base/kg per houri with electrocardiographic monitoring. |
- Very few areas now have chloroquine sensitive malaria (Figure XXX1)
- Areas where the partner drug to artesunate is known to be effective
- Artemisinin derivatives are not registered in the USA and some other temperate countries
- Fixed dose co-formulated combinations are now available
- Tetracycline or doxycyline should not be given in pregnancy or to children under 8 years.
- Oral treatment should be substituted as soon as the patient recovers sufficiently to take fluids by mouth.
- Artesunate is the drug of choice when available. The data from large studies in South East Asian showed a 35% reduction in mortality compared with quinine. Severe malaria in children in high transmission settings has different characteristics, and so trials are ongoing in Africa comparing artesunate with quinine to determine whether there is a survival benefit in African children.
- A loading dose should not be given if therapeutic doses of quinine or quinidine have definitely been given in the previous 24 h. Some authorities recommend a lower dose of quinidine.
- Infusions can be given in 0.9% saline, 5% or 10% dextrose/water. Infusion rates for quinine should be carefully controlled.

The treatment of falciparum malaria has changed radically in recent years. In endemic areas the World Health Organization now recommends artemisinin-based combinations (ACTs) as first line treatment for uncomplicated falciparum malaria. These rapidly and reliably effective drugs are often unavailable in temperate countries such as the United States where treatment recommendations are limited by the registered available drugs. In August 2007, the Food and Drug Administration (FDA) gave the Centers for Disease Control and Prevention (CDC) permission to provide intravenous artesunate for severe malaria. The availability of antimalarial drugs varies considerably between countries. Fake or adulterated drugs, including antimalarial agents, are being sold in many low-income countries.
Severe Malaria
In large studies conducted in Asia, parenteral artesunate, a water-soluble artemisinin derivative, has been shown, compared to quinine, to reduce the mortality of severe falciparum malaria by 35%. It has therefore become the drug of choice. Artesunate is given intravenously but can also be administered by intramuscular injection. A rectal formulation of artesunate has been developed as a community-based pre-referral treatment in the rural tropics for patients unable to take oral mediations. These drugs are also safer than quinine and are considerably safer than quinidine but they are not yet available in the United States.
The antiarrhythmic quinidine gluconate is as effective as quinine and, as it is more readily available, has replaced quinine for the treatment of malaria in the United States. Quinine is safer than quinidine; cardiovascular monitoring is not required except when the recipient has cardiac disease.
Parenteral antimalarial treatment should be started as soon as possible. If artemether, quinine, or quinidine are used, an initial loading dose must be given so that therapeutic concentrations are reached as soon as possible. Both quinine and quinidine will cause dangerous hypotension if injected rapidly; when given intravenously, they must be administered carefully by rate-controlled infusion only. If this is not possible, quinine may be given by deep intramuscular injections to the anterior thigh. It has been recommended that -- if safe and feasible -- exchange transfusion should be considered for patients with severe malaria but there is no clear evidence it is beneficial.
When the patient is unconscious, the blood glucose level should be measured every 4 to 6 h and values <2.2 mmol/L (40 mg/dL) should mandate treatment with intravenous dextrose. Anemia develops rapidly; if the hematocrit falls to <20%, then whole blood (preferably fresh) or packed cells should be transfused slowly, with careful attention to circulatory status. Children presenting with severe anemia and acidotic breathing are often hypovolemic; in this situation, resuscitation with crystalloids or blood is indicated.
Management of fluid balance is difficult in severe malaria, particularly in adults, because of the thin dividing line between overhydration (leading to pulmonary edema) and underhydration (contributing to renal impairment). If necessary, central venous pressures should be measured and maintained in the low-normal range. As soon as the patient can take fluids, oral therapy should be substituted for parenteral treatment.
Uncomplicated Malaria
Infections from P. vivax, P. malariae and P. ovale> should be treated with oral chloroquine (total dose, 25 mg of base/kg). In much of the tropics, drug resistant P. falciparum has been increasing in distribution, frequency and intensity. Chloroquine-resistant P. falciparum is now present throughout most of the tropical world and resistance to sulfadoxine/pyrimethamine is becoming widespread.
To prevent resistance, falciparum malaria should be treated with drug combinations and, in endemic areas, no longer with single drugs. This combination strategy is based upon simultaneous use of two or more drugs with different modes of action: one, usually an artemisinin derivative (artesunate, artemether or dihydroartemisinin), given for 3 days; and the other, a slower-acting antimalarial to which P. falciparum is sensitive. Artemisinin combination treatments are now first line recommended treatment for falciparum malaria.
In multidrug resistant areas (parts of Asia and South America) either artemether-lumefantrine or artesunate-mefloquine should be used. Although significant resistance to mefloquine has been documented in Thailand, Myanmar, Vietnam and Cambodia, mefloquine is usually effective against multidrug resistant strains of P. falciparum outside these areas and, in combination with artesunate, achieves cure rates over 90% nearly everywhere. Atovaquone-proguanil is also highly effective. In more drug sensitive areas either of these drugs, or artesunate-amodiaquine, or artesunate- sulfadoxine/pyrimethamine can be used depending on the prevailing drug susceptibility pattern.
As second line drugs for the treatment of recrudescence following first line treatment, artesunate or quinine plus tetracycline, doxycycline or clindamycin given for seven days are all effective. Tetracycline and doxycycline cannot be given to pregnant women or to children <8 years of age. Oral quinine is extremely bitter and regularly produces cinchonism comprising tinnitus, high-tone deafness, nausea, vomiting and dysphoria.
If there is any doubt as to the identity of the infecting malarial species, treatment for falciparum malaria should be given. Nonimmune subjects receiving treatment for malaria should have daily parasite counts performed until negative thick films indicate clearance of the parasite. If the level of parasitemia does not fall below 25% of the admission value in 48 h or if parasitemia has not cleared by 7 days (and adherence is assured), drug resistance is likely and the regimen should be changed. If treatment failures occur with commonly used antimalarial agents, alternative drugs should be used (Table 5).
-resistant P. falciparum is now present throughout most of the tropical world and resistance to sulfadoxine/pyrimethamine is becoming widespread.

Table 5. Alternate Drugs for Use When Initial Malaria Treatment Fails.
| Drugs Used Initially | Drugs Use to Treat Recrudescence |
|---|---|
| Chloroquine | Sulfadoxine/pyrimethaminea |
| Sulfadoxine/pyrimethamine | Artesunate-mefloquinea |
| Mefloquine±artesunate | Quinine±tetracycline (or doxycycline) for 7 days |
a = Or artemether-lumefantrine

To eradicate persistent liver stages and prevent relapse (radical treatment), primaquine (0.25-0.5 mg of base/kg; adult dose) should be given daily for 14 days to patients with P. vivax or P. ovale infections after laboratory tests for G6PD deficiency have proved negative.
Complications
The blood glucose level should be checked regularly thereafter, as recurrent hypoglycemia is common, particularly in patients receiving quinine or quinidine. Patients who develop spontaneous bleeding should be given fresh blood and intravenous vitamin K. Convulsions should be treated with intravenous or rectal benzodiazepines and, if necessary, respiratory support. Aspiration pneumonia should be suspected in any unconscious patient with convulsions, particularly with persistent hyperventilation; intravenous antimicrobial agents and oxygen should be administered, and pulmonary toilet should be undertaken. Hypoglycemia or Gram-negative septicemia should be suspected when the condition of any patient suddenly deteriorates for no obvious reason during antimalarial treatment.
Prevention
These are halcyon days for malaria prevention and control. New drugs have been discovered and developed; highly effective drugs, insecticide-treated nets and insecticides for spraying dwellings are being purchased for endemic countries by the Global Fund for HIV/AIDS, Malaria and Tuberculosis and the President's Malaria Initiative. The Roll Back Malaria Partnership, Global Health Council and others are advocating strongly for even greater support.
Still, the eradication of malaria is not yet feasible because of the widespread distribution of Anopheles breeding sites; the great number of infected persons; the continued use of ineffective antimalarial drugs; and inadequacies in trained operational and research workers and material resources, infrastructure and control programs.
Malaria may be contained by judicious use of insecticides to kill the mosquito vector, rapid diagnosis and appropriate patient management, and administration of intermittent presumptive treatment or chemoprophylaxis to high-risk groups. Malaria researchers are intensifying their efforts to understand parasite-human-mosquito interactions better and to develop more effective control and prevention interventions.
Despite the enormous investment in efforts to develop a malaria vaccine, no safe, effective, long-lasting vaccine is likely to be available for general use in the near future. While there is promise for one or more malaria vaccines on the more distant horizon, prevention and control measures continue to rely on antivector and drug use strategies.
Personal Protection Against Malaria
Simple measures to reduce the frequency of mosquito bites in malarious areas include the avoidance of exposure to mosquitoes at their peak feeding times (usually dusk and dawn), but also throughout the night, and the use of insect repellents containing DEET (10 to 35%) or picardin (7%) if DEET is unacceptable, suitable clothing, and insecticide-impregnated bed nets or other materials. Widespread use of bed nets treated with residual pyrethroids (preferably of long-duration) reduces the incidence of malaria in areas where vectors bite indoors at night and has been shown to reduce mortality in western and eastern Africa.
Chemoprophylaxis
Recommendations for prophylaxis depend on knowledge of local patterns of plasmodial drug sensitivity and the likelihood of acquiring malarial infection. When there is uncertainty, drugs effective against resistant P. falciparum should be used [atovaquone-proguanil (Malarone®), doxycycline, mefloquine or primaquine] (see Table 6).
Chemoprophylaxis is never entirely reliable and malaria should always be considered in the differential diagnosis of fever in patients who have traveled to endemic areas, even if they are taking prophylactic antimalarial drugs.
Pregnant women traveling to malarious areas should be warned about the potential risks. All pregnant women at risk in endemic areas should be encouraged to attend regular antenatal clinics. Apart from mefloquine, the safety of other prophylactic antimalarial agents in pregnancy has not been established.
Antimalarial prophylaxis has been shown to reduce mortality in children between the ages of 3 months and 4 years in malaria endemic areas but it is not a logistically or economically feasible option in many countries. The alternative, to give intermittent treatment doses [intermittent presumptive treatment (IPT)] shows promise for more widespread use in infants, young children and pregnant women. Children born to non-immune mothers in endemic areas (usually expatriates moving to malaria endemic areas) should receive prophylaxis from birth.
Travelers should start taking antimalarial drugs at least 1 week before departure so that any untoward reactions can be detected and therapeutic antimalarial blood concentrations will be present when needed. Antimalarial prophylaxis should continue for 4 weeks after the traveler has left the endemic area, except if atovaquone-proguanil or primaquine has been taken -- these drugs have significant activities against the liver stage of the infection (causal prophylaxis) and can be discontinued 1 week after departure from the endemic area.
Atovaquone-proguanil (3.75/1.5 mg per kg or 250/100 mg, daily adult dose) is a fixed-combination once-daily prophylactic agent that is very well tolerated by adults and children, with fewer adverse gastrointestinal effects than chloroquine-proguanil and fewer adverse central nervous system effects than mefloquine. Atovaquone-proguanil is best taken with food or a milky drink to optimize absorption. There are insufficient data on the safety of this regimen in pregnancy.
Mefloquine (250 mg of salt weekly, adult dose) has been widely used as an antimalarial prophylactic because it is usually effective against multidrug-resistant falciparum malaria and is reasonably well tolerated. Mild nausea, dizziness, fuzzy thinking, disturbed sleep patterns, vivid dreams and malaise are relatively common. Approximately 1 in every 10,000 recipients develops an acute reversible neuropsychiatric reaction manifested by confusion, psychosis, convulsions or encephalopathy.
Daily administration of doxycycline (100 mg daily, adult dose) is an effective alternative to atovaquone-proguanil or mefloquine. Doxycycline is generally well tolerated but may cause vulvovaginal thrush, diarrhea and photosensitivity and cannot be used by children <8 years old or by pregnant women.
Chloroquine remains the drug of choice for the prevention of infection with drug-sensitive P. falciparum (now found in very few areas of the world) and with the other human malarial species (Figure 2). Chloroquine is generally well tolerated, although some patients are unable to take the drug because of malaise, headache, visual symptoms (from reversible keratopathy), gastrointestinal intolerance or (in dark-skinned patients) pruritus. Chloroquine is safe in pregnancy.
With chronic administration for >5 years, a characteristic dose-related retinopathy may develop but this condition is rare at the doses used for antimalarial prophylaxis. When used continuously, amodiaquine, a related aminoquinoline, is associated with a high risk of agranulocytosis (~1 person in 2,000) and also hepatotoxicity (~1 person in 16,000) and should not be used for prophylaxis.
Primaquine (0.5 mg of base/kg or 30 mg, daily adult dose taken with food) has proved safe and effective in the prevention of drug-resistant falciparum and vivax malaria in adults. This drug can be considered for travelers to areas with or without drug-resistant P. falciparum and who are intolerant to other recommended drugs. Abdominal pain and oxidant hemolysis, the principal adverse effects, are not common as long as the drug is taken with food and is not given to G6PD-deficient persons. Primaquine should not be given to pregnant women or neonates.
Government Resources
Because of the increasing spread and intensity of antimalarial drug resistance, the Centers for Disease Control and Prevention (CDC; http://www.cdc.gov/malaria/index.htm), which recommends a daily dose of atovaquone-proguanil for all travelers, maintains an updated 24-h travel and malaria information audiotape that can be accessed by touch-tone telephone (877-FYI-TRIP).
Regional and disease-specific documents may be requested from the CDC Fax Information Service (888-232-3299). Consultation for the evaluation of prophylaxis failures or treatment of malaria can be obtained from state and local health departments and the CDC Malaria Hotline (770-488-7788) or the CDC Emergency Operations Center (770-488-7100).
Further Readings
Baird JK, Schwartz E, Hoffman SL. Prevention and treatment of vivax malaria. Curr Infect Dis Rep. 2007;9:39-46.
Breman JG, Alilio MS, Mills A. The intolerable burden of malaria II. What's new, what's needed: a summary. Am J Trop Med Hyg 2004; 71(2,3 Suppl):1-11.
Dondorp A et al, and the South East Asian Quinine Artesunate Malaria Trial (SEAQUAMAT) group. Artesunate versus quinine for treatment of severe falciparum malaria: a randomised trial. Lancet 366, 717-725. 2005.
Centers for Disease Control and Prevention: Treatment of malaria. (Guidelines to clinicians). Atlanta, Department of Health and Human Services, 2007 http://www.cdc.gov/malaria/pdf/clinicalguidance.pdf (accessed 10 June 2007).
White NJ: The assessment of antimalarial drug efficacy. Trends Parasitol 18:865, 2002.
White NJ, Breman JG. Malaria. In Harrisons Principles of Internal Medicine, 17th ed., D Kasper, E Braunwald, AS Fauci, SL Hauser, DL Longo, JL Jameson, eds., McGraw Hill Co., New York, 2007, in press.
World Health Organization: Severe falciparum malaria. Trans R Soc Trop Med Hyg 94(Suppl 1):51, 2000.
World Health Organisation. Guidelines for the treatment of malaria. WHO, Geneva, 2006.
World Health Organisation Assessment and Monitoring of Antimalarial Drug Efficacy for the Treatment of Uncomplicated Falciparum Malaria. WHO/HTM/RBM/2003.50.