Course Authors
Frederick Sweet, Ph.D.
Frederick Sweet, Ph.D. is Professor of Reproductive Biology in Obstetrics and Gynecology, Washington University School of Medicine, St. Louis, Missouri.
Dr. Sweet reports no commercial conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the diverse concepts that constitute targeted therapy
Describe recent basic and clinical progress from research on these novel therapies
Discuss how near or far we are from having more effective new therapies for ovarian cancer "just around the corner."
Ovarian cancer is the fifth leading cause of death from all cancers in women in the United States. Even though overall survival figures have shown improvement during the past few decades with the introduction of new chemotherapy schedules, the majority of patients die of this disease. The relatively new research area of targeted therapy offers promise and hope for more effectively treating ovarian cancer than has been obtained by traditional approaches. Thus the main purpose of this Cyberounds® on targeted therapy is to provide an overview of this rapidly evolving field as it progresses from the research laboratory to the oncology clinic.
The basic concept of targeting therapies against invasive microorganisms and also cancers has been around for more than a century, summarized under Father of Targeted Therapy (below). We almost take for granted the effective targeted therapies against invasive microorganisms, antibacterial and antifungal antibiotics, which are on virtually every pharmacy shelf worldwide. Hopefully soon, we shall have on those shelves more effective anticancer drugs, promised to be just around the corner in reviews such as this one.
Father of Targeted Therapy
The magic bullet concept -- a term first coined at the turn of the 20th century by Paul Ehrlich (1854-1915) -- derives from 19th century German chemists who developed synthetic dyes for selectively staining tissues in histological examination. Some dyes were discovered to be particularly well suited for staining bacteria. The young Ehrlich had been an exceptionally gifted histological chemist who discovered the classical precursor technique to Gram staining bacteria.
Ehrlich had proposed that if a compound could be made that selectively targeted a disease-causing organism -- analogous to how certain dyes selectively stain bacteria -- then a toxin for that organism could be delivered by linking it to the chemical agent that selects the tissue. Meanwhile, in the early and mid-1890s, he worked with his friend Emil Adolf von Behring on developing the diphtheria serum.
In 1896, Ehrlich was made director of the new Institute of Serum Research and Examination [Institut für Serumforschung und Serumprüfung in Steglitz (Berlin)]. The next year, Ehrlich's work on serum led to his famous side-chain theory (Seitenkettentheorie). His theory explained the effects of serum and enabled detection of an antigen. Then in 1899, Ehrlich's Institute was moved to Frankfurt (Main) and extended into the Royal Institute of Experimental Therapy (Institut für experimentelle Therapie) where he continued research on infectious diseases and chemotherapy.
Describing targeted therapy, Ehrlich for the first time used the English expression "magic bullet" in his Harben Lectures.(1) The German word Zauberkugel (comparable to the Freikugel of Weber's opera Der Freischütz) had appeared earlier in his thoughts and publications, based on his "side-chain" theory views. This was also the precursor of our present concept of receptors. Ehrlich had set the standard that a useful drug must not harm the host while it attacks the parasitic invader.
In 1906, Ehrlich discovered the structural formula of atoxyl, a fairly toxic chemical compound that had been shown to be useful in treating sleeping sickness. Following this discovery, he tried to create a less toxic version of this substance. Then in 1908, Ehrlich (together with Ilya Ilyich Mechnikov) received the Nobel Prize in Medicine for his development of effective therapeutics.
However, Ehrlich's first rationally designed magic bullet was Salvarsan (or arsphenamine) discovered with his student Sahachiro Hata, the year after Ehrlich received his Nobel Prize. Salvarsan provided the only cure for syphilis until the mid 20th century. It was replaced after World War II by penicillin and others of the new family of antibiotics.
Ehrlich was first to propose attaching toxins to antibodies that can carry a deadly substance to the site of an invading parasite (or cancer) while sparing normal tissues. His proposal anticipated, indeed inspired development of today's immunotherapy. This concept has been adopted by virtually all modern laboratories using monoclonal antibodies as carriers for analogs of anthracyclines (e.g., daunorubicin), paclitaxel or cisplatin for treating ovarian cancer by this form of targeted therapy.
NIH's Bernhard Witkop notes:(2) "...in the fifth Paul Ehrlich Lecture at the NIH in 1992, Manfred Eigen (Nobel Prize 1967, Paul Ehrlich Prize 1995), spoke about interfering with intercellular transfer of information as a new kind of magic bullet against viral infections. Finally, genes as therapeutic agents are now in the forefront of pharmaceutical research, and promise to be of help in diseases that appeared incurable."
Witkop concluded: "Subsequent speakers who developed "magic bullets" were George Hitchings (1905-1998; Nobel Prize 1988, who lectured in 1990) and Manfred Eigen (Nobel Prize 1967, who spoke in 1992, the same year in which he received the Paul Ehrlich Prize). Stanley Prusiner lectured in 1995, receiving the Paul Ehrlich Prize in the same year, and the Nobel Prize in 1997 for his discovery of prions, dormant 'magic bullets'."
Immunotherapy
Ehrlich had predicted that a promising delivery system for carrying an anticancer drug to its target while bypassing normal tissues would be based on immunology. But such a delivery system required discovering a unique cell surface protein in the targeted cancer. The unique protein would serve as an antigen from which to produce the complementary antibody to serve as a drug carrier.
Excellent progress has been made in developing new treatments for ovarian cancer by using as a delivery system the antibody against the antigen CA125. Originally, the ovarian cancer cell surface antigen had been -- and is still -- used for detecting the CA125 antigen in the blood of women suspected of having ovarian cancer.(3) Also, measuring decreasing blood levels of the antibody during chemo- and/or radiation therapy provides a means of assessing therapeutic progress as the ovarian cancer undergoes remission.
Employing the anti-CA125 antibody in a delivery system for targeted therapy requires using very large amounts (i.e., milligrams to grams) of the antibody protein, compared with its routine use (i.e., ng to μg amounts) for measuring the ovarian cancer antigen in women's blood. Moreover, effective and very efficient chemical methods had to be devised for linking a cancer therapeutic agent to the antibody in this type of a drug delivery system.
Immunotoxins
The potential, practical downsides are that chemical modification of cancer therapeutic agents (e.g., daunorubicin, paclitaxel, cisplatin, etc.) for their linkage to an antibody often reduces their potency. Earlier, work from our laboratory with immunoconjugates of daunorubicin linked to an anti-CA125 antibody emphasized that previously such attempts had been unsuccessful because modifications of the drug weakened or destroyed its efficacy.(4)
Similarly, when therapeutic agents are linked to an antibody (anti-CA125), the location of the linkage in the immunoglobulin often reduces the specificity and/or its avidity for the targeted ovarian cancer cell surface antigen (Figure 1). Thus a great deal of experimentation goes into obtaining an optimized configuration before an antibody-drug complex can show promise for targeted therapy.

Figure 1. Idealized Antibody (Ab)-drug Conjugate.
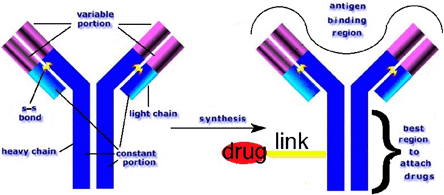
Schematic of the functional structures in a typical antibody protein (left) and regions where anticancer therapeutic drugs should and should not be linked (right). Even today, most Ab-drug conjugates are formed by randomly linking a drug to the Ab protein -- tending to reduce specificity and avidity by drug molecules occupying the antigen-binding region.

Radioimmunotherapeutics
More recently, a radioisotope-antibody conjugate was administered together with paclitaxel (i.e., paclitaxel) in Phase I trials for treating ovarian cancer by radioimmunotherapy (RIT).(5) A mouse-derived monoclonal antibody, CC49, which targets an ovarian cancer-associated glycoprotein (TAG-72) was conjugated with an analog of 90Y to provide the resulting radioimmunoconjugate 90Y-CC49 (administered with an initial dose of 14 mCi/m2 90Y). At the same time, paclitaxel was administered i.p. to the ovarian cancer patients. The 20 patients treated ranged in ages from 39 to 77 years (median age, 61 years).
Discussion of the results with the radioimmunoconjugate 90Y-CC49 concluded: "partial responses were noted in 2 of 11 patients (18%) with measurable disease treated in the current trial. In addition, the disease-free interval exceeded 12 months in five of nine patients with non measurable disease, and four of these five patients remain cancer free 15+ to 23+ months after treatment."
"This experience provides evidence that this strategy can be administered safely, is well tolerated and has antitumor activity. It also provides solid evidence that IFN α2b enhancement of target antigen expression and i.p. administration of substantial dosages of paclitaxel (100 mg/m2) are well tolerated and do not preclude administration of RIT at maximum or near maximum doses in a combined multimodality format."
More recently, further refinements in combining radioimmunotherapy with paclitaxel in nude mice established that this approach indeed has a synergistic anti-tumor effect on the growth of human ovarian tumor OVCAR-3 xenografts.(6) Synergy was only observed when a tumor-specific antibody was used as a delivery system for radioimmunotherapy. In this case, the combination of radioimmunotherapy using an anti-CA125 monoclonal antibody and chemotherapy with paclitaxel was shown to be effective in an in vivo model of ovarian cancer that may hold promise as a treatment regimen for patients with ovarian cancer.
A Practical Clinical Problem
There is an important practical clinical problem lurking around immunotherapy that is rarely discussed as part of the optimistic conclusions about immunotherapy in the recent literature (or for that matter in NIH grant applications). In two words the problem is antigen shedding. What enables the anti-CA 125 monoclonal antibody to detect and measure the cell surface antigen from ovarian cancer is that the cancerous tumor sheds the CA125 antigen that is detected in the patient's blood.
When the same anti-CA125 monoclonal antibody is incorporated into an anticancer immunotoxin, its function is to carry and target the toxin for bonding to ovarian cancer cells producing CA125 cell surface antigens. Presumably, most molecules of the shed, blood borne CA125 antigenic protein would become bound to and thus neutralize the immunotherapeutic agent. Clearly, antigen shedding constitutes a major problem of a practical clinical nature. For efficacy of immunotherapy, this problem must be solved. The author has speculated on various strategies for dealing with this problem but they are beyond the scope of this Cyberounds®.
Originally, the term targeted therapy had been applied to substances that were a throwback to Paul Ehrlich's magic bullet concept. Purists still regard targeted therapy to derive from a binary molecular structure analogous to military intercontinental ballistics missiles. Part of the molecule is the carrier and cancer-seeking vehicle, while the other moiety is a cytotoxin or lethal radioisotope. More recently, however, the concept has been broadened to encompass targeting entire systems, discussed in the following sections (below).
Targeting p53
Immunological research during the past decades revealed that ovarian cancer patients exhibit significant immune responses against their tumors. Out of this work, it has been discovered that a protein named p53 is a tumor-associated antigen that may be promising as a target in ovarian cancer.(7) This possibility comes from an increased knowledge about the role of the immune system in ovarian cancer.
The interaction between the host immune system and ovarian cancer tumor cells is still not completely understood. However, several observations suggest that cell-mediated immune responses may be important in controlling ovarian cancer.
An attractive tumor specific antigen in ovarian cancer is the frequently overexpressed, mutated p53 tumor suppressor protein. [Other possible target antigens like HER-2/neu and MUC-1 are less frequently expressed by ovarian tumor cells.] The role of p53 (and other cancer genes) has been extensively reviewed.(8) P53 acts as a transcription factor, playing a key role in coordinating cell cycle arrest, DNA repair and apoptosis following DNA damage to promote genomic stability.
P53, as a transcription factor, mediates apoptosis by pathways involving the upregulation of pro-apoptotic genes and also downregulation of anti-apoptotic genes. It also has the capacity to induce apoptosis directly from the cytoplasm via direct activation of Bax, causing mitochondrial permeability. This releases cytochrome c, leading to the induction of apoptosis.
In cancer cells, loss of wild-type p53 function can lead to more aggressive tumor growth and failure to respond to standard therapy. Most commonly, loss of function is through mutation. P53 is one of the most commonly mutated tumor suppressor proteins in human tumors.
Phase I/II immunization trials using p53 as an antigen were carried out. In the Phase I study, six advanced stage cancer patients were immunized with an adenoviral vector encoding wild type p53.(9) Although all patients showed an adenoviral immune response, neither tumor responses nor anti-p53 responses were observed. It was concluded that the strong anti-adenoviral specific response may have limited a p53-specific response in humans.
However, based on results in the mouse system and rhesus macaques, a phase I/II clinical study involving vaccination of end-stage colorectal cancer patients with a recombinant canary pox virus (ALVAC) encoding wild type p53 was performed.(10) These patients were immunized intravenously with increasing doses of ALVAC-p53. From this study, it appeared that this modality is safe and capable of stimulating p53-specific immune responses in several of these patients. One out of 16 patients showed stable disease for a short period of time after immunization with the highest dose. Fever had been the only vaccine related adverse effect. The authors conclude from this trial that repeated immunizations are probably necessary.
The role of p53 as tumor antigen for targeting ovarian cancer by immunotherapy-based trials continues to evolve. As the important issues of safety and immunogenicity of vaccination strategies are progressing. Clinical effectiveness is, of course, the major aim of future trials with this mode of targeted therapy.
Targeting the Coagulation Cascade
Coagulation factors are reported to exert a profound effect on tumor cell behavior based on both in vivo and in vitro studies.(11) These factors enhance tumor cell proliferation, invasion, angiogenesis and metastasis. Hence, targeting activated coagulation factors potentially provides yet another strategy for treating ovarian cancer.
Blood coagulation and kallikrein-kinin systems consist of plasmatic proteolytic cascades. These cascades participate in the initial host response against foreign components and in wound healing. The increased expression of factor XII in the peritoneum tissue of ovarian cancer patients(12) suggests the intrinsic coagulation cascade becomes activated and may very well be involved in ovarian cancer cell progression. Indeed, recent clinical data suggest that disrupting certain elements of the coagulation and inflammation processes in the tumor microenvironment may provide a new approach to cancer therapy.
Heparins are the most extensively used anticoagulants in clinics. In blood coagulation, unfractionated heparin and low-molecular-weight heparins potentiate the activity of antithrombin III. This, in turn, inhibits the activation of coagulation factors II and X.(13) Heparins also release TFPI, a physiologic inhibitor of the TF pathway that prevents pulmonary embolism and is used to treat deep vein thrombosis.(14)
Retrospective and meta-analytic studies of deep vein thrombosis treatment have shown longer survival among cancer patients with thrombosis treated with unfractionated heparin and low-molecular-weight heparins than among patients treated without heparin.(15) Thus, the use of anticoagulants could possibly extend their lives.
There is a higher incidence of venous thromboembolism in patients with melanoma or small cell lung cancer. A pilot study of enoxaprin, a low-molecular-weight heparin for the treatment of advanced melanoma was reported but the clinical outcome was somewhat ambiguous.(16) Then, a phase II trial of combination chemotherapy and anticoagulant therapy (docetaxel plus enoxaparin) was performed in chemotherapy-naïve, metastatic non-small cell lung cancer patients.(17)
The median time to progression was five months and the median survival time was 11 months. The most common toxic effects were neutropenia and asthenia; no clinically significant bleeding or thrombotic events were reported. Treatment was well tolerated in patients with advanced small cell lung cancer with the results suggesting that enoxaparin could prolong the time to disease progression.(17) Both the oral anticoagulant warfarin and unfractionated heparin have been shown to prolong survival time among patients with small cell carcinoma.(18)
A prospective, randomized, controlled trial cancer patients (N=385) into two arms: placebo and dalteparin (a low-molecular-weight heparin). The cancers presented included breast, colorectal, ovarian and pancreatic.(19) Thirty-four percent (34%) of the dalteparin group and 31% of the placebo group received chemotherapy alone, 8% of each group received radiation therapy alone; the remainder received radiation and chemotherapy. Estimated overall survival at one, two and three years was not significantly different between the groups. But the estimated overall survival among patients with a better prognosis at enrollment (55 patients in the dalteparin group and 47 in the placebo group) was significantly longer in the dalteparin group at two years (78% and 60%, P<0.03) and at three years (55% and 36%, P<0.03).
A second study confirmed the antineoplastic effects of dalteparin.(20) During a 12-month followup period of the study, 602 patients with solid tumors and venous thromboembolism were randomly assigned to a dalteparin or coumarin-derivative treatment group. Among patients without metastatic disease, the probability of death at 12 months was 20% in the dalteparin group compared with 36% in the oral anticoagulant group (P<0.03). In patients with metastatic cancer, no difference in mortality was observed between the treatment groups. Nevertheless, the effects of dalteparin on survival were statistically significant between patients with and without metastatic disease (P<0.02).
Although the exact mechanism by which heparin mediates antitumor or anti-metastatic activity is not yet known it merits further study as a potential targeted therapy.
Targeted Gene Therapy
An important rationale for mapping the human genome had been that elucidating the molecular biology of carcinogenesis would lead to the introduction of gene therapy for the treatment of ovarian and other cancers. Clearly, cancer must develop from altered genetic processes in normal cells. A growing body of evidence suggests that targeting these defects may offer some advantage towards suppressing tumor growth and proliferation. But the field of gene therapy is so large that it could easily occupy scores of Cyberounds®. Thus the present section can only hope to give the reader a small sampling of the field while keeping it focused on ovarian cancer.
Manipulating abnormally altered cellular processes via gene therapy promises to be useful for treating a variety of human cancers, among them ovarian malignancies.(21) Development of a strategy for gene-targeted therapy is somewhat analogous to those developed for immunotherapy except, instead of antibodies doing the carrying and targeting, viruses take over that function in gene therapy. Thus targeted gene therapy is based on using an adenoviral vector to selectively deliver a genetically encoded toxin that possesses selective expression in cancer cells. The more specific the delivery of genetic material to cancer cells, the greater the therapeutic index. Thus, molecular biologists have been developing methods for improving cancer cell specificity by both direct and also indirect approaches.

Figure 2. Schematic Representation of Gene Therapy with an Adenovirus Vector.
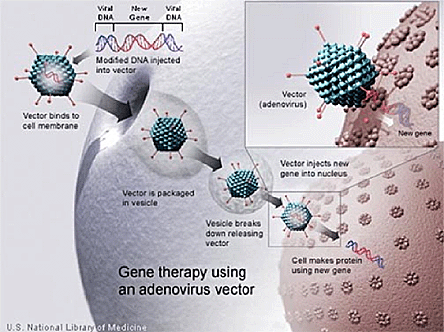
Courtesy of the National Library of Medicine.

The direct approach involves delivering a toxin-encoding gene to cancers for inducing specific genetic changes in tumor cells. These changes then would promote the cancer cellular uptake of a drug (e.g., to which the cancer may have developed a resistance). The specificity of the subsequent tumor destruction can occur by transduction targeting of the toxin delivered specifically to the tumor cell.
Another means of specificity is "transcriptional targeting" that involves tumor specific transcriptional activators for turning on a toxin gene. Thus for example, a retroviral vector (LNISN) has been used to transduce a rodent sodium/iodide symporter gene (rNIS) within cancer cells. The receptor-expressing gene enables the cancer cells to selectively bind radioactive substances such as 123I for tumor imaging, or 131I for gamma-induced, selective cell lysis. Administering 131I to the transduced cancer cells results in tumor death, similar to the mechanism by which the isotope causes thyroid ablation.(22)
Overcoming Multiple-Drug Resistance?
The effect of chemotherapy on cancer cells is a dose-dependent relationship. Nevertheless, certain epithelial cancers exhibit significant resistance to multiple chemotherapeutic agents. Further studies on these cancers led to discovering a drug efflux pumping glycoprotein (p170) produced by the MDR1 cDNA gene.(23)
Because a dose-limiting factor for many chemotherapeutic agents is bone marrow toxicity, researchers investigated how this chemotherapy-resisting pump offers protection. Studies on the transduction of MDR1 gene into hematopoietic cells demonstrated a degree of resistance and, therefore, protection from unwanted side effects.(23),(24) But this effect was not long-lasting as a result of short-lived gene expression and low levels of protein production in vivo.(24)
At least one research group has demonstrated some success in overcoming the limitation by extending cell cultures to multiple exposures before reimplantation. Eventually, protein expression lasted for up to two years.(25) Although evidence of transduction 10 weeks following treatment was seen in only 2 out of 5 patients, a Phase I trial showed this treatment to be safe and feasible.(26)
Other studies have proposed the addition of a pro apoptotic gene with the MDR1 gene in order to kill any residual tumor cells in the sample prior to transduction of bone marrow cells. This would improve the efficacy of the therapy and overcome the potential problem of transducing tumor cells with the MDR1 gene increasing their drug resistance.(27)
What Is Slowing Clinical Developments?
So far, very little progress has been made towards developing clinically useful ovarian cancer-targeted gene therapy. Certain problems confound trying to translate some spectacular laboratory results into overcoming poor infectivity and poor specificity. Part of the problem is that most adenoviral vectors and CRAds are Adenovirus serotype 5-based that, alas, preferentially bind to coxsackie-adenovirus receptor (CAR). CAR expression is highly variable in ovarian cancer cells, and limits the oncolytic potential and effectiveness of the most promising gene therapy agents.(28)
However, it has been well established that adenoviral fiber modifications can enhance the infectivity and thereby improve the transductional targeting of CRAds. Additionally, by employing tumor specific promoters, viral replication can be restricted to tumor cells, thus improving specificity via transcriptional targeting. CRAds created with dual targeting modalities hold promise for adenoviral agents that are ovarian cancer-specific, and can potentially overcome previous challenges towards producing clinical benefits.
Yet another stumbling block to the clinical application of gene therapy is analogous to the antigen shedding problem that impedes the successful use of immunotherapy (described under the section on Immunotherapy.) Several laboratories have demonstrated that ovarian malignant ascites contains neutralizing anti-adenovirus antibodies.(29) Presumably, the presence of these vectors in ascites prevents successful gene transformation of the targeted cancer cells.
Although previous studies suggest that a partial escape of viruses from preexisting anti-Ad antibodies can occur via fiber modifications or chimerism,(30) there is an uncertainty surrounding gene transduction. Even though biopsies are reliable, they are not practical because of the large numbers of biopsies that are needed to determine the characteristics of transgene expression. Therefore, noninvasive imaging to detect the level, persistence and location of the treatment vector or tumor is important for evaluating the effectiveness of gene therapy. Rapidly developing technology in radiologic diagnosis makes the feasibility of imaging in gene therapy virtually certain.
Indeed, noninvasive imaging techniques will become increasingly important in the development of more effective targeted gene therapies. Undoubtedly, as more specific vectors are developed, gene therapy will offer a promising approach for treating women with ovarian cancer.