Course Authors
Eve Van Cauter, Ph.D.
Dr. Van Cauter is Professor of Medicine, University of Chicago School of Medicine, Chicago, IL.
Within the past 12 months, Dr. Van Cauter has been a consultant to Pfizer, Sanofi-Aventis and Unilever, a member of the scientific advisory board of Select Comfort and a major shareholder of SloWave Inc.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss how contemporary lifestyles in industrialized societies are associated with a reduction in sleep time due to multiples causes
Discuss the relationship between a state of sleep debt and important adverse effects on risk of obesity and diabetes
Discuss the role played by the orexin system in linking sleeping and feeding.
Sleep loss can occur as a result of voluntary short bedtimes or from the presence of a pathological condition that is associated with reduced total sleep time. Behavioral sleep curtailment and reduced sleep duration and/or quality from sleep disorders are both increasingly prevalent in modern society. While sleep has long been considered to be important for the brain but not for the rest of the body, there is rapidly increasing evidence that insufficient sleep duration and/or poor sleep quality have adverse effects on peripheral physiology and may increase the risk of obesity and diabetes, as well as compromise immune function.
Prevalence of Sleep Loss in Modern Society
Sleep curtailment is a hallmark of modern society that is often considered harmless, efficient and laudable. Socio-economic constraints and the availability of leisure and work opportunities around the clock have contributed to promote short sleep times. In industrialized societies, roughly 20% of the work force is engaged in shift work and these work schedules generally involve a substantial sleep loss.
The decrease in sleep duration seems to have developed over the past four decades. In 1960, a U.S. survey of over one million individuals found a modal sleep duration of 8.0-8.9 hours.(1) The polls conducted by the National Sleep Foundation in 2000, 2001 and 2002 indicated that the average American sleeps 6.9-7.0 hours.(2) Overall, sleep duration thus appears to have decreased by 1.5 to 2 hours during the second half of the 20th century. Conversely, the time spent awake has increased from 15.5-15.6 hours in the early seventies to 17.2-17.3 hours at the beginning of the new millennium.(3) In 2004, more than 30% of adult men and women between the ages of 30 and 64 years reported sleeping less than 6 hours per night.(4) A recent study has evaluated sleep duration in nearly 700 adults ages 38-50 years by wrist actigraphy rather than self report.(5) The average sleep duration was 6.1 hours. (The actigraph is a small unit, usually attached to the wrist, which continuously records gross motor activity.)
Sleep deprivation also occurs in children and adolescents. In 2006, the National Sleep Foundation presented the findings of a survey of 1,602 children aged 11-17 years living in the continental U.S.(2) While the sleep need of adolescents has been estimated to be around 9 hours,(6) average sleep duration was only 8.4 hours in 6th graders and decreased steadily with each subsequent grade to only 6.9 hours in 12th graders. An amazing 28% of high school students admitted falling asleep at school at least once a week.
Intriguingly, the dramatic increase in the incidence of obesity and diabetes seems to have developed over the same period of time as the progressive decrease in self-reported sleep duration. The two secular trends mirror each other over the second half of the 20th century.(7)
Chronic sleep loss may also be secondary to pathological conditions, including insomnia and obstructive sleep apnea (OSA). One of the consequences of the aging of the population is a higher prevalence of insomnia. Chronic insomnia is estimated to affect 10-15% of the population.(8) In middle-aged adults, OSA has been reported to affect 24% of men and 9% of women.(9) Data from the 2005 Sleep in America poll of the National Sleep Foundation indicate that as many as one in four adults, and more than 50% of obese individuals, are at high risk for OSA.(2) While OSA involves sleep fragmentation, respiratory disturbances and hypoxic stress in addition to sleep loss per se, reduced total sleep time is also a component of this condition.
Taken together, sleep loss, either behavioral or disease-related, affects millions of individuals in our modern society. Recent laboratory and epidemiologic studies have provided evidence indicating that sleep loss may have adverse health consequences. These studies have focused on three important components of physical health:
- glucose homeostasis and diabetes risk;
- appetite regulation and obesity risk and
- immune function.
This Cyberounds® will review the evidence for an adverse effect of reduced total sleep time on these three axes.
Impact of Reduced Sleep Duration on Glucose Metabolism and Diabetes Risk
Blood levels of glucose are tightly regulated within a narrow range to avoid hypoglycemia and hyperglycemia, as both conditions have adverse life threatening consequences. Glucose tolerance refers to the ability to metabolize exogenous glucose and return to baseline blood glucose levels. Glucose regulation is markedly different during sleep as compared to waking hours.(10)
The first detailed laboratory study that examined the effects of recurrent partial sleep deprivation on glucose metabolism involved healthy young men who were subjected to 6 nights of 4 hours in bed ("sleep debt") followed by 7 nights of 12 hours in bed ("sleep recovery").(11) At the end of each bedtime condition, the subjects underwent an intravenous glucose tolerance test (ivGTT) and a 24-hour period of frequent blood sampling. The data from the ivGTT were analyzed using a mathematical model(12) that allows for the derivation of critical parameters contributing to glucose metabolism. Table 1 summarizes the findings.

Table 1. Effects of Recurrent Partial Sleep Deprivation.
| Fully rested | After 5 days of sleep restriction | p level | |
|---|---|---|---|
| KG (% per minute) | 2.40 ± 0.41 | 1.45 ± 0.31 | <0.04 |
| AIRg (μU.ml) | 548 ± 158 | 378 ± 136 | 0.05 |
| SI (104.min1.(μU/ml) | 6.73 ± 1.24 | 5.41 ± 0.60 | 0.28 |
| Disposition Index | 2897 ± 404 | 1726 ± 395 | 0.0006 |

The initial release of insulin following glucose injection, referred to as the "acute insulin response to glucose" (AIRG), was 30% lower when the subjects were in the state of sleep debt than when they were fully rested. A trend for reduced insulin sensitivity (SI), suggesting that higher amounts of insulin were needed to metabolize the injected glucose bolus, was also evident but failed to reach statistical significance. The analysis of glucose and insulin levels during an ivGTT also allows for the derivation of a validated marker of diabetes risk, the so-called disposition index (DI), which is the product of AIRG x SI.
DI values of 2,000 and above are typical of subjects with normal glucose tolerance, while DI values under 1,000 have been found in populations at high risk for Type 2 diabetes. Compared to the after sleep recovery group, those with sleep debt had a 40% lower DI and 3 of the 11 subjects had DI values under 1,000. Consistent with the reduced DI, the rate of glucose clearance post-injection was 40% slower at the end of the sleep debt condition as compared to the recovery condition. The profiles of glucose and insulin levels following breakfast ingestion were in agreement with the results of the ivGTT, with higher glucose levels despite similar levels of insulin after short, as compared to long, sleep.
Taken together, the findings indicated that glucose metabolism in these young lean adults submitted to less than one week of sleep restriction was similar to that typical of older adults with impaired glucose tolerance, a pre-diabetic state. The findings of this first "sleep debt" study were confirmed in a second study that examined the impact of sleep restriction (4 hours per night for 2 nights) as compared to sleep extension (10 hours per night for 2 nights) using a randomized cross-over design.(13)
The results of these laboratory studies stimulated epidemiologic research on the possible link between diabetes risk and short and/or poor sleep in existing cohort studies. One limitation common to all these epidemiologic studies is that sleep variables were assessed by self-report. Seven prospective studies, originating from Germany, Japan, Sweden and the U.S., have been published so far and six of them reported a significant association between low sleep duration and/or quality and the subsequent development of Type 2 diabetes after controlling for multiple possible confounders [reviewed in (14)]. The only negative study originated from Sweden and included the smallest number of subjects (N=600).(15)
The possibility that there could be an association between short sleep duration and the severity of an existing diabetic condition was addressed in a recent survey study. Self-reported sleep duration, sleep quality and hemoglobin A1c levels, a key marker of glucose control, were examined in African Americans with Type 2 diabetes.(16) Perceived sleep debt was calculated as the difference between preferred sleep duration and actual weekday sleep duration. After controlling for age, gender, BMI and insulin use, it was found that, in patients without diabetic complications, levels of hemoglobin A1c were associated with perceived sleep debt but not sleep quality. In contrast, in patients with at least one complication, hemoglobin A1c was associated with sleep quality but not perceived sleep debt. The magnitude of the effects of sleep duration or quality was comparable to that of widely used oral anti-diabetic drugs. While these associations suggest that improving sleep duration and/or quality in Type 2 diabetes may improve glycemic control, the direction of causality cannot be inferred from the associations found in this cross-sectional study.
The mechanisms underlying alterations in glucose metabolism following recurrent partial sleep restriction are likely to be multifactorial. Sleep loss has been linked with decreased brain glucose metabolism, increased sympathetic nervous activity, elevated levels of evening cortisol, increased secretion of ghrelin and growth hormone during waking hours and increased levels of pro-inflammatory cytokines. All of these factors could potentially adversely affect glucose homeostasis.
Impact of Reduced Sleep Duration on Appetite Regulation and Obesity Risk
Appetite is regulated by neural mechanisms that are influenced by metabolic and hormonal signals.(17),(18) The arcuate nucleus of the hypothalamus includes two opposing sets of neuronal circuitry, an appetite-stimulating center and an appetite-inhibiting center. Among the peripheral signals that affect these neuronal regions are leptin, a satiety hormone, and ghrelin, an orexigenic hormone. Leptin is produced by the adipocytes and is the major long-term signal informing the brain of the status of energy stores in fat tissue. Low leptin levels promote food intake. Ghrelin is released primarily from the stomach. In rodent models, ghrelin promotes a positive energy balance and increased adiposity through increased food intake and reduced fat oxidation.(19) Studies in humans indicate that ghrelin is partly responsible for mealtime hunger and meal initiation. Plasma ghrelin levels are rapidly suppressed by food intake and then rebound after 1.5-2 hours, paralleling the resurgence in hunger. Thus, leptin and ghrelin exert opposing effects and have been referred to as the "ying and yang" of appetite regulation.(20)
Sleeping and feeding are fundamentally related. Indeed, wakefulness is needed to sustain feeding and sleep is invariably associated with fasting. Sleep deprivation in animals occurs mainly when there is a shortage of food and extended wakefulness is needed to continue foraging.(21) The human may well be the only mammalian species that voluntarily engages in sleep deprivation in the absence of an emergency situation. Rodents submitted to total sleep deprivation for prolonged periods of time increase markedly their food intake.(22)
The identification in the lateral hypothalamus and erifornical area of a population of neurons that express two excitatory neuropeptides (orexin A and orexin B, also referred to as hypocretin A and hypocretin B) that have potent wake promoting effects and also stimulate food intake, has provided a molecular basis for the interactions between feeding and sleeping.(23),(24) Orexinergic neurons project directly to major wake-promoting centers in the brain stem and in the hypothalamus, and activate appetite-promoting neurons in the arcuate nucleus. Orexinergic activity is modulated by peripheral signals, with glucose and leptin exerting inhibitory effects, while ghrelin promotes further activation.(24)
In animal models, experimental sleep deprivation -- which invariably involves increased activity and/or stress -- results in increased orexinergic activity.(25) It is not known whether sleep deprivation in humans under comfortable sedentary conditions, e.g., in an armchair in front of a television, is similarly associated with overactivity of the orexin system.
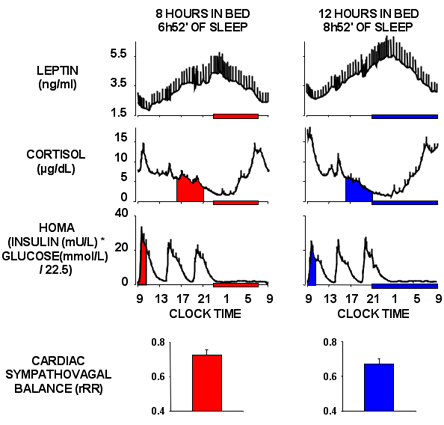
There is evidence indicating that sleep restriction in humans results in a dysregulation of the neuroendocrine control of appetite, promoting hunger in the absence of caloric need. Two studies of 1-3 days of total sleep deprivation showed a reduction in the amplitude of the 24-hour variation of leptin levels.(26),(27) In the "sleep debt" study that involved 6 days of 4-hour bedtimes followed by 6 nights of extended recovery sleep, leptin concentrations were clearly lower at the end of sleep restriction than at the end of the recovery period.(28) These changes in leptin occurred despite similar levels of caloric intake and physical activity and no change in body weight between the two bedtime conditions. The difference in leptin levels between the state of sleep debt and the fully rested state corresponded roughly to the impact of three days of dietary intake restriction by approximately 900 Kcal per day. The 24-h profile of circulating leptin levels appears to be exquisitely sensitive to sleep duration -- reducing sleep duration by as little as 2 hours lowers the levels of this satiety signal, as illustrated in the upper panel of Figure 1.
In a recent epidemiologic study involving more than 700 adults,(29) leptin levels in adults who reported being short sleepers (5-6 hours per night) were significantly lower than expected for their degree of adiposity.

Figure 1. Hormone Levels and Cardiac Sympathetic Activity in Sleep Debt and Extended Recovery Sleep.

A laboratory study using a randomized cross-over design assessed not only leptin levels but also ghrelin levels, subjective hunger and appetite at frequent intervals after 2 night of sleep restriction (4-h per night) or extension (10-h per night) under strictly controlled conditions of constant caloric intake.(30) Consistent with previous studies, the levels of leptin decreased by 18% during sleep restriction. Furthermore, the levels of the hunger-promoting signal, ghrelin, increased by 28%. On average, the neuroendocrine "hunger to satiety ratio" (i.e., ghrelin-to-leptin) increased by 70%.
Ratings of subjective humger and appetite were higher during sleep restriction than sleep extension and the change in the ratio of ghrelin-to-leptin between the two conditions was strongly correlated to the change in hunger ratings. This suggests that the observed hormonal changes were at least partially responsible for the increased hunger experienced by the subjects when their bedtime was curtailed. When sleep-restricted, the subjects reported craving for carbohydrate-rich foods (+32%) more than for foods of other macronutrient composition (+18%), possibly because the "tired" brain craves glucose, its major fuel.
A population-based study, the Wisconsin Sleep Cohort Study, also observed an association between sleep duration, leptin and ghrelin in more than 1,000 individuals.(31) Average nightly sleep was calculated from sleep diaries and, in addition, each subject underwent one night of laboratory sleep recording. On awakening in the laboratory, a single blood sample was obtained for the measurement of hormonal levels. The results indicated that short sleep in the laboratory was associated with higher morning ghrelin levels, while short nightly sleep at home was associated with lower leptin levels independently of BMI. Despite major differences in subject population and study design, the findings of the laboratory study(30) and of the Wisconsin Sleep Cohort study(31) were in remarkable agreement and suggested that sleep loss may alter the ability of leptin and ghrelin to accurately signal caloric need, acting in concert to produce an internal perception of insufficient energy availability.
Between 1992 and 2007, 13 epidemiologic studies in adults and 8 in children have reported a negative association between sleep duration and BMI, with short sleepers having higher BMI after controlling for a number of possible confounders.(14),(29) Of note, the association was observed in studies that enrolled subjects with different BMI, from lean or mildly overweight to frankly obese, and that originated from different countries and cultures. Most studies were cross-sectional in design but two studies in adults and three in children exploited a longitudinal design and were consistent -- indicating a higher risk of weight gain with short sleep [reviewed in (14)]. While the epidemiologic evidence for an association between short sleep and obesity is rapidly accumulating, it should be noted that nearly all studies, whether in adults or children, relied on subjective reports of sleep duration.
Taken together, the evidence from laboratory and epidemiologic studies has raised the possibility that partial chronic sleep deprivation, a novel condition that seems to have become widespread in modern societies and particularly in the U.S., may play a role in the current epidemic of obesity. The increased prevalence of "sleep debt" has been considered as the number one "nontraditional" explanation for the epidemic of obesity, after the "two big" traditional causes, i.e., excessive caloric intake and decreased physical activity.(3) Of note, the evidence reviewed here suggests that sleep loss may promote excessive food intake. Another intriguing possibility that needs to be addressed is that individuals in a state of sleep debt may have less incentive to be physically active and may favor sedentary obesigenic behaviors.
Impact of Sleep Deprivation on Immune Function
Evidence is accumulating to support the view that sleep is important for immune function, particularly in exerting a positive influence on the initial steps of an adaptive cellular immune response.(32) Conversely, sleep loss may have a detrimental impact on the immune system. Findings that sleep deprivation elevates the circulating levels of inflammatory markers such as interleukin-6, tumor necrosis factor α and C-reactive protein suggest that the risk or severity of a wide spectrum of medical conditions that involve chronic inflammation (e.g., cardiovascular disease, diabetes, arthritis) could be increased by sleep loss.
There is also evidence for an important role for sleep for efficient immune defense, which supports the popular wisdom that not getting enough sleep increases an individual's chance of catching a cold or other ailments. One study examined the antibody response to hepatitis A vaccination over a 28-day period in healthy young subjects who either had a normal night of sleep following inoculation or were kept awake all night.(33) Antibody titers post-vaccination were nearly 50% lower in those who had been sleep deprived as compared to those who had a normal night of sleep. Another study examined the response to influenza vaccination after 4 consecutive nights of partial sleep deprivation (bedtimes of only 4 hours per night) and continued bedtime restriction for 2 more nights, as compared to immunization preceded and followed by normal sleep durations.(34) Figure 2 illustrates the findings.

Figure 2. Antibopdy Titers in Sleep Deprived and Normal Sleep Subjects.
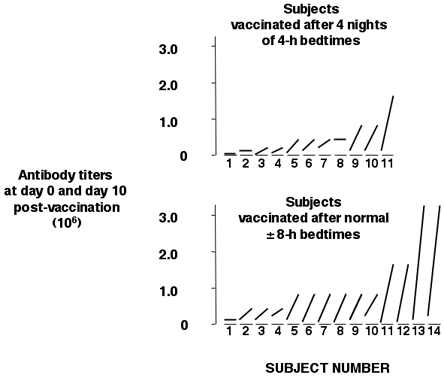

On average, antibody titers on the 10th day after immunization were also reduced by half in those who had been sleep-deprived as compared to those with normal sleep. Thus, the two studies concur and support an adverse effect of sleep loss on the adaptive immune response. This finding suggests that prior sleep history may be considered in optimizing vaccination programs. It is also possible that some of the well-documented adverse effects of stress on immune function could be mediated by sleep loss and that the reductions in sleep duration and quality that are typical of aging may be involved in the reduced efficacy of vaccines in older populations.
Conclusions
Clearly, sleep is not only for the brain but also for the rest of the body. Recent evidence suggests that sleep loss, a highly prevalent -- and often strongly encouraged -- condition in modern society could be a risk factor for major chronic diseases, including obesity and diabetes.
Figure 1 illustrates some of the most salient effects of recurrent partial sleep deprivation on peripheral systems. It may be seen that reduced leptin levels, increased evening cortisol levels, higher degree of insulin resistance (as estimated by the HOMA index), higher levels of cardiac sympathetic nervous activity and increased subjective sleepiness occur in healthy lean young adults submitted to a very modest degree of sleep restriction. Most of these alterations are markers of allostatic load, i.e., the cumulative wear and tear on body systems caused by too much stress and/or inefficient management of the systems that promote adaptation.(35) Although in the comfortable and unchallenging environment of the laboratory, chronic sleep deprivation is not subjectively perceived as psychologically stressful(28) by research participants, the biological findings indicate that sleep deprivation activates stress-responsive physiologic systems and could be viewed as a chronic stressor.