Course Authors
Eric Siu, M.Sc., Nael Al Koudsi, H.B.Sc., Man Ki Ho, H.B.Sc., Rachel F. Tyndale, M.Sc., Ph.D.
Dr. Tyndale is a Scientist at the Centre for Addiction and Mental Health and a Professor of Pharmacology, University of Toronto. Mr. Siu, Mr. Al Koudsi, and Miss Ho are doctoral students in the Department of Pharmacology at the University of Toronto.
Within the past 12 months, Dr. Tyndale has been the Chief Scientific Officer and a shareholder of Nicogen Inc. Mr. Siu, Mr. Al Koudsi and Miss Ho report no commercial conflicts of interest.
This activity is made possible by an unrestricted educational grant from Pfizer. ![]()
Estimated course time: 3 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the epidemiology of smoking among U.S. population
Discuss the neurophysiological basis of nicotine addiction, including the role of the reward pathway
Discuss the genetic contribution to smoking behaviours and how genetic variation in neurotransmitter pathways might contribute
Discuss pharmacotherapeutic treatment strategies that promote smoking cessation.
Dr. Tyndale and colleagues will discuss the unlabeled use of rimonabant and NicVAX for smoking cessation.
According to the World Health Organization (WHO), tobacco smoking is responsible for the death of approximately 5 million people each year.(1) If current trends continue, by the year 2030 it is estimated that 10 million people will die per year as a result of smoking.(2) In the United States alone, tobacco use accounts for approximately $157 billion in health care costs and annual loss of productivity.(3)
In spite of increasingly strict tobacco control policies and widespread knowledge of the numerous adverse health effects associated with cigarette smoking, approximately 21% of adults in the United States are currently smokers (Figure 1).(4),(5) One of the national health objectives is to lower smoking prevalence to under 12% in adults by the year 2010, and while there has been an overall decline in recent years it is not occurring at a rate that will allow this objective to be met.(6)
Large differences in smoking prevalence exist among American racial/ethnic groups, with American Indians/Alaska Natives having the highest rates at 33.4%, followed by non-Hispanic whites (22.2%) and non-Hispanic blacks (20.2%), with the lowest rates found among Hispanics (15.0%) and Asians (11.3%).(4) The gender gap in smoking prevalence has dramatically narrowed in recent years, with overall current rates of 23.4% in men and 18.5% in women (Figure 1).(2),(4),(5)

Figure 1. The Change in Smoking Prevalence in Men and Women From 1955 to 2005.*
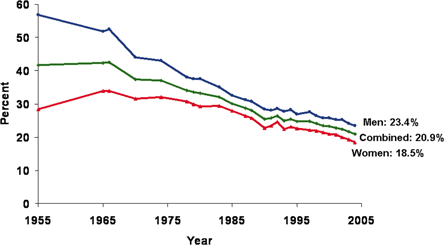
*Cokkinides, V. et al. Progress and opportunities in tobacco control. CA Cancer J Clin 2006;56(3):135-142.

Neurobiology of Nicotine Dependence
While the majority (~80%) of smokers express a desire to quit smoking, less than 5% are able to maintain abstinence unaided as a consequence of the highly addictive properties of inhaled cigarette nicotine, the primary psychoactive substance in cigarettes.(7),(8) Cigarettes, unlike other nicotine delivery systems (e.g., patch or gum), are particularly addictive because smoked nicotine is rapidly absorbed and reaches the brain (site of action) within 10-20 seconds;(9) the rapid delivery intensifies addictiveness at least in part by enhancing the learning of the behavior.
In the brain, nicotine mediates its psychoactive properties, which include mood elevation, decreased anxiety, decreased appetite, increased arousal and cognitive enhancement, by stimulating nicotinic acetylcholine receptors (nAChRs).(10) Nicotinic AChRs are pentameric ligand, ion gated-channels containing combinations of α (α2 - α7) and β (β2 - β4) subunits that form either homomeric (e.g., α7 nAChR) or heteromeric (e.g. α4β2 nAChR) receptors that differ in their affinity for nicotine.(11),(12)
Of the many nAChR subunits, α4 and β2 subunits are the most widely expressed, high affinity subunits in the brain.(13) Animal studies indicate that the activation of the α4 subunit is required for nicotine-induced reward, tolerance, and sensitization,(14) whereas the β2-containing nAChRs are involved in mediating the reinforcing properties of nicotine but not the expression of nicotine withdrawal symptoms.(15),(16) These studies showed that distinct molecular and neural mechanisms mediate differing components of nicotine dependence.
The main mechanism by which nicotine is thought to establish and maintain dependence is by activating the mesocorticolimbic dopamine system, also referred to as the brain reward center (Figure 2).(11),(17) Nicotine activates nAChRs on dopaminergic neurons in the ventral tegmental area (VTA) that innervate the nucleus accumbens (NAc), resulting in sustained dopamine release.(17),(18) Other addictive drugs (e.g., cocaine) and natural rewards (e.g., sex) also promote dopamine release in the NAc. In addition, because of the predominantly post-synaptic localization of the nicotinic receptors, nicotine modulates the release of other neurotransmitters such as serotonin and γ-aminobutyric acid (GABA) which can have direct psychoactive properties (e.g,. serotonin) and indirect effects on dopamine release.(19)

Figure 2. The Mesocorticolimbic Dopamine System.
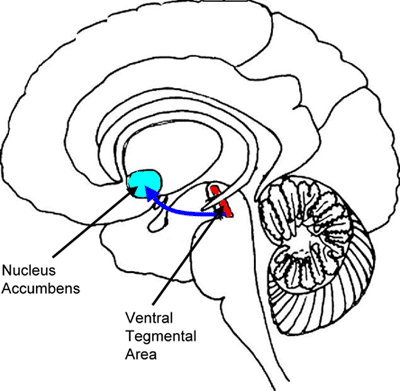

The Genetics of Nicotine Addiction
Cigarette smoking is a complex behavior that includes a number of stages such as initiation, experimentation, regular use, dependence, cessation and relapse.(20),(21) While environmental factors such as the influence of family members, peers and culture undoubtedly affect one's smoking behaviors at these stages, genetics also plays a substantial role. Twin studies have been used as a tool to determine the heritability of complex traits such as smoking. The degree of concordance of a given phenotype between genetically identical monozygotic twins compared to dizygotic twins, who share only approximately half of their genes, is an indication of a genetic contribution to that phenotype.(22)
From numerous twin studies, the heritability of "ever" smoking has been estimated in the range of 11-78%.(23),(24),(25),(26),(27),(28),(29),
(30),(31) This large variation may be due, at least in part, to the broadness of this phenotype, which encompasses those who have only tried cigarettes once to regular heavy smokers. Once smoking is initiated, the heritability for persistence to regular smoking has been estimated at 28-84%,(23),(26),(27),(28),(30),(31),(32),(34) for number of cigarettes smoked per day at 45-86%(33),(34),(35),(36),(37),(38),(39) and for dependence on nicotine at 31-75%.(30),(34),(40) A genetic influence on nicotine withdrawal symptoms and smoking cessation has also been identified with heritability estimated at 26-48% and 50-58% respectively.(33),(41),(42),(43) Taken together, these studies suggest a substantial genetic contribution to most aspects of smoking including the ability to quit smoking.
While heritability studies can demonstrate a genetic influence in smoking behaviors, advances in our understanding of the human genome have allowed for the localization of specific genes involved. Similar to other complex disorders, multiple interacting genetic factors are likely involved in the etiology of smoking behaviors, with each gene contributing a small effect.(34),(44),(45) Genome-wide linkage analyses are used to locate areas in the genome with high linkage disequilibrium to a specific phenotype such as cigarette consumption.
Some of the loci associated with smoking phenotypes are near genes of known biological relevance, including the α2-nicotinic acetylcholine receptor (CHRNA2) and α1A-adrenergic receptor (ADRA1A) on chromosome 8, the dopamine receptor (D1) on chromosome 5, the μ1-opioid receptor (OPRM1) on chromosome 6 and the serotonin receptor 5A on chromosome 7.(46) Genes present in loci identified from these genome-wide linkage studies, as well as those with biological relevance, can be further examined for their impact on smoking using, for example, case-control association studies.
Nicotine's addictive properties are a result of the activation of nAChRs in the brain and the consequent impact on a number of downstream neurotransmitter systems (e.g., dopamine, serotonin and GABA). Therefore, genes encoding receptors, transporters and enzymes involved in the synthesis or degradation of these neurotransmitters are plausible candidates for gene association studies with smoking phenotypes. Here we provide a few examples of gene association studies and briefly discuss some of the discrepancies in the literature. For an extensive review see (44),(47),(48).
Dopaminergic System
The dopaminergic system has been studied extensively because of its pivotal role in mediating nicotine dependence. Of the five different dopamine receptor subtypes (D1-D5), a single nucleotide polymorphism 10 kb downstream of the DRD2 gene (TaqI A1 allele),which encodes the dopamine D2 receptor, has received considerable attention. The TaqI A1 variant lies within a neighboring gene (ANKK1) and is linked to another variant in the D2 receptor (TaqI B1).(49),(50),(51) TaqI A1 allele has been associated with reduced dopamine D1 receptor expression in the striatum,(52),(53),(54) an increased risk for current smoking, an earlier age of smoking onset and shorter abstinent periods from smoking. (55),(56) However, other studies have shown conflicting results.(57),(58),(59)
Interestingly, the TaqI A1 allele has also been shown to influence abstinence rates on nicotine replacement therapy (NRT) and bupropion(60),(61),(62) Johnstone et al. (2004) found that transdermal nicotine patch is significantly more effective than placebo for smokers with at least one TaqI A1 allele.(61) A follow-up of this study supported the association between TaqI A1 and cessation at 6 and 12 months of follow up but only among women.(62) In a recent bupropion cessation study, Swan et al. (2005) reported a trend for lower cessation rates (at 12-month follow up) among women with the TaqI A1 allele.(60) Thus, it seems that women with the TaqI A1 allele might benefit more from NRT compared to bupropion.
Another interesting genetic variant (-141C Ins/Del) in DRD2 has also been associated with different cessation rates for NRT and bupropion. Individuals homozygous for the -141C allele had higher abstinence rates on bupropion (35%) compared to placebo (19%), while those with the (-141C Ins/Del allele had higher abstinence rates on nicotine nasal spray (42%) or patch (50%) compared to placebo (33%) or bupropion (20%).(63) This is an example of:
- how response to specific treatments may be differentially altered by genetic variation in the same gene and
- how genetics could potentially be used to improve cessation by individualizing treatment based on genotype (e.g., improving efficacy by treating individuals with the -141C Ins allele with bupropion and those with the -141C Del allele with NRT).
Other important players in the dopaminergic system include the dopamine transporter and enzymes involved in either the synthesis (e.g., tyrosine hydroxylase) or metabolism/degradation (e.g., dopamine β-hydroxylase, monoamine oxidase and catechol-O-methyltransferase) of dopamine. A number of studies have found significant associations between several smoking phenotypes (e.g., craving or dependence) and the genes encoding the dopamine transporter(64),(65),(66) and neurotransmitter enzymes.(67),(68),(69),(70),(71)
Nicotinic Receptors
Li et al. (2005) recently reported an association between genetic variations in CHRNA4, the gene encoding the α4 subunit of the nAChR, with the following phenotypes: smoking quantity, heaviness of smoking index or Fagerstrom test for nicotine dependence.(72) However, there are no reported significant associations between genetic variations in CHRNB2, the gene encoding the β2 subunit of nicotinic receptor, and nicotine dependence.(72),(73),(74),(75)
Other Candidate Genes
The serotonin and the opioid systems have both been shown to play an important role in nicotine dependence.(76),(77) Thus, it is not surprising that associations have been reported between genetic variants in genes encoding serotonin or opioid receptors with different smoking phenotypes and cessation rates.(78),(79) For example, a polymorphism (102T>C) in the serotonin receptor 2A (5-HT2A) gene has been associated with lower 5-HT2A expression in the brain(80) and current smoking in a Brazilian population.(79)
Limitations and Significance of Genetic Association Studies
Lack of replication in genetic association studies is still common for a number of reasons such as:
- Racial admixture: the inclusion of individuals of different ethnicities can influence the results since different ethnic groups have unique genetic variants and differing frequencies of variants.
- Inconsistency among studies in genotype assessment and phenotypes (e.g., smokers versus nicotine dependence) being measured.
Nonetheless, gene-association studies provide insight into the neurobiological mechanisms of nicotine dependence and provide the rationale for future use of genetics to identify individuals who might be at a greater risk for smoking or smoking related illnesses. In addition, pharmacogenetics holds the promise of improving the efficacy of smoking cessation treatments by individualizing the type, dosage and duration of treatment based on an individual's genotype/phenotype.
Established Drug Treatments
Nicotine Replacement Therapies
Nicotine replacement products (NRTs) are one of the first-line treatments for nicotine dependence approved by the US Federal Drug Administration (FDA). Currently, there are several nicotine delivery devices available on the market - these include gum, transdermal patch, vapor inhaler, nasal spray, lozenge and sublingual tablet. NRT treatments facilitate cessation by delivering nicotine without the exposure to carcinogenic compounds found in cigarette smoke. The primary mechanisms of action of NRTs is to maintain the desirable emotional states (e.g., mood, attention) achieved from smoking and reduce withdrawal symptoms associated with abstinence by providing steady state amounts of nicotine.(81),(82) The use of nicotine replacement products has led to varying degrees of success in long-term smoking cessation.(83),(84) Overall, NRTs increase the odds for abstinence by 1.5- to 2-fold compared to placebo at 6 months after target quit date.(84) In addition, various types of NRTs appear to be equally effective and behavioral counseling does not substantially improve cessation rates beyond the NRT effect.(84) More extensive discussions on each of the NRTs can be found elsewhere.(85),(86),(87)
Bupropion (Zyban®)
Both smoking and depression are highly associated in that they are influenced by dopamine levels.(88),(89) The first non-nicotine product approved as a first-line therapy for smoking cessation is the atypical anti-depressant bupropion. The main mechanism of action of bupropion in smoking cessation appears to be related to craving reduction and alleviation of certain symptoms associated with withdrawal in abstinent smokers; however, other less understood mechanisms may also be involved.(90),(91),(92),(93)
Bupropion reduces negative withdrawal symptoms which may be mediated by binding to striatal dopamine transporters and preventing the reuptake of dopamine.(94),(95) Bupropion can also potentially attenuate the rewarding effects of nicotine by acting as an indirect functional antagonist of the nAChRs.(96),(97),(98) Though the precise mechanism of action is unclear, bupropion can also act on the noradrenergic system where it reduces the firing rates of noradrenergic neurons in the locus coeruleus,(99) which contains neuronal projections to the hippocampus, a region implicated in drug-dependence.(100) Finally, bupropion metabolites, in particular hydroxybupropion, have been demonstrated to be be pharmacologically active, although the individual effects of these metabolites on nicotine dependence have not been clarified.(101),(102),(103)
Overall, most studies have demonstrated bupropion to be effective at improving smoking cessation(91),(104),(105),(106),(107),(108),(109),
(110),(111)(112) while some studies showed no significant improvement compared to placebo;(113),(114) (113, 114)[see review (108)]. With respect to safety, a large amount of data have been gathered over the past few years concerning the use of bupropion for smoking cessation in a community setting. The drug caused few serious side effects with the primary reasons for discontinuation being insomnia, nausea/vomiting and dizziness.(115)
Drugs in Development
Varenicline (ChantixTM)
Varenicline has recently been approved by the FDA as a new smoking cessation drug. It is a partial agonist of the α4β2 nAChR; its structure is based on the alkaloid extract cytisine from the plant Cytisus laburnum L.(116) Varenicline is hypothesized to reduce nicotine reward during smoking and to stimulate dopamine release to reduce craving and withdrawal during abstinence.(116),(117) Two separate Phase III 12-week treatment studies found that varenicline more than doubled the continuous abstinence rates up to one year compared to placebo.(118),(119) Varenicline was also found to be significantly more efficacious in maintaining continuous abstinence up to one year compared to bupropion.(118),(119) The most common adverse event related to varenicline was nausea.(118),(119)
Nicotine Vaccines
Nicotine vaccines are a new approach being investigated for smoking cessation. The principle of this strategy is to stimulate antibody production which will prevent nicotine from entering the brain.(120),(121) An advantage of the nicotine vaccines is that daily drug administration is not required. A peer-reviewed safety study found that the 30-day continuous abstinence rates were 4-fold higher in patients receiving the highest vaccine (NicVAX) dose.(122) In a phase II study, Cytos Biotechnology reported that high responders (high antibody production) were twice as likely to be continuously abstinent from smoking compared to placebo at 6 and 12 months. Careful interpretation of these results is needed as sample sizes were small in both studies. Most of the reported side effects were mild to moderate and were resolved without medical treatment.(122),(123)
Rimonabant (SR141716)
Rimonabant is a cannabinoid receptor antagonist.(124) In animal studies it was found that rimonabant prevented intravenous nicotine self-administration and blocked the release of dopamine in response to nicotine.(124) In a phase III study (STRATUS-US), it was found that patients receiving the highest dose of rimonabant had the greatest abstinence rate compared to those who were on a lower dose or placebo.(125) In contrast, another study (STRATUS-Europe) found no significant increase relative to placebo in cessation for either dosing group.(126) In a third study (STRATUS-Worldwide), significantly more patients who received the highest dose remained smoke-free for the entire year compared to placebo.(126) The most common side effects were nausea and upper respiratory tract infection.(125) Rimonabant has not been approved for use as a smoking cessation product in the USA and the moderate benefits obtained from this drug may limit its widespread use.
Metabolism Modulators
Another novel approach to smoking cessation is through inhibition of nicotine metabolism. In humans the majority of nicotine is metabolically inactivated to cotinine by the CYP2A6 enzyme.(126),(127) Alterations in the amount or function (e.g., due to genetic variations or functional inhibition) of this enzyme significantly affect nicotine metabolism.(128),
(129)(130),(131) As smokers titrate their smoking to maintain nicotine levels(8),(132),(133) variation in the activity of the enzyme can alter smoking behaviors;(134),(135),(136),(137),(138),(139),(140) inhibiting CYP2A6 might represent a novel approach to smoking cessation and smoking reduction products.
Summary
Nicotine is the primary psychoactive substance responsible for establishing and maintaining tobacco dependence. Like other drugs of abuse (e.g. cocaine), nicotine acts via the mesolimbic dopaminergic pathway stimulating feelings of reward. Genetic linkage studies have identified a host of genes (e.g. nicotinic receptor genes) that may contribute to the variable risk for tobacco dependence. The first-line pharmacotherapies for tobacco dependence include nicotine replacement therapies, bupropion (Zyban®) and the newly approved varenicline. In addition, genetic studies may provide additional therapeutic targets for smoking cessation and opportunities in the future for optimization of smoking cessation therapy based on genetics.