Course Authors
Val Shestopalov, Ph.D.
Dr. Shestopalov is Assistant Professor, Bascom Palmer Eye Institute, Department of Ophthalmology, Department of Cell Biology and Anatomy University of Miami Miller School of Medicine, Miami, Florida.
Dr. Shestopalov reports no commercial conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the basic biology and physiology of the lens
Diagnose and classify cataracts
Conduct a systematic evaluation of patients with cataracts
Discuss the relationship between cataracts and systemic medical conditions.
The spatial and temporal patterns of congenital cataracts are typically consistent upon the expression of mutant form of one of the genes implicated in the lens development. In a similar manner, alterations of critical structural elements of the lens during development and maturation of the lens impede multiple intrinsic mechanisms of resistance to oxidative and mechanical damage and, ultimately, affect the tissue transparency in adults. Accumulation of damage and general decrease in efficiency of stress-resistance cause age-related cataract, the most common cause of blindness worldwide.
Mechanisms of Resistance to Ageing
Although most cells in the adult lens are devoid of organelles and become quiescent, it is a mistake to treat this tissue as dead. The lens maintains layers of transcriptionally active cells and possesses active mechanisms for homeostasis, preserving proteins from photooxidation and aggregation throughout an individual's life. This is achieved through four major mechanisms:
- actively lowering oxygen content in the lens center
- synthesis of potent anti-oxidants scavenging reactive oxygen species
- synthesis of large amounts of molecular chaperones preserving protein from aggregation and precipitation
- constant internal circulation of water, ions and metabolites.(1),(2)
These functions are performed by the lens epithelium and outer cortical fibers that represent a protective outer layer of the vertebrate lens.(3)
These potent protective mechanisms become challenged after trauma and during aging (affecting lens homeostasis in people over the age of 50). A variety of adverse changes converges to impact the aging lens homeostasis. In addition to the accumulation of oxidized aggregated proteins and lipids, chaperones (soluble alpha crystallins) and anti-oxidants (glutathione, ascorbate, catalase, vitamin E) become depleted and internal microcirculation of ions and small metabolites is significantly reduced. Experimental evidence indicates that a barrier forms between the active superficial layers and the quiescent core of the lens, which restrains diffusion of essential anti-oxidants and molecular chaperones, and regeneration of the protective molecular machinery in the lens nucleus.(4),(5) This implies that the formation of the most prevalent cataract type, the age-related (senile) cataract, stems from a transport problem affecting the lens nucleus and facilitating oxidative damage to proteins and lipids.(6),(7)
Cataract Classification
The term cataract refers to opacification of the crystalline lens in the human eye. This is the most common cause of visual loss in humans and has been well known since early civilizations. The ancient Egyptians described cataracts as "darkening of the pupil" and "white disease of the eye."(8)
Currently, there is no unified system of cataract classification. They are classified either by morphological appearance, color intensity (for senile cataracts), localization and type of opacities or, which is common in clinical practice, by etiology and age of onset.(9) Alternatively, classification can be made according to pathological mechanisms disrupting normal lens biology. The former approach subdivides cataracts into infantile (juvenile), inherited and systemic.(10)
The pathological classification may refer to the type of damage to lens cells and the way the opacity is thought to occur, as well as to the origin of light-scattering particles. The presence of parallel pathological processes complicates classification and necessitated introduction of a broad systemic category that includes most common age-related cataracts affecting half of all the U.S. population over age 80. In the U.S. alone, more than 20 million people of age over 40 either have a cataract or have had cataract surgery. By origin, cataracts can be broadly divided into congenital, environmental and age-related, where the latter type is the most common worldwide.
Congenital Cataracts
Congenital cataract can be infantile and early postnatal, both familial and non-familial. Although quite rare in developed countries, this category is of particular importance because of its high impact on an individual's lifelong visual acuity and its potential to cause amblyopia, squint and nystagmus. Prevention of visual impairment secondary to congenital cataract is an important component of the World Health Organization's (WHO) international program for the elimination of avoidable blindness by 2020.
Congenital cataracts commonly result from alterations of the early developmental events or by infections like rubella virus. Early fetal development is influenced by genes coding for transcription factors (e.g., Pax6, Pitx3, Maf or Sox). If the maturing lens is affected, mutations are commonly found in the genes encoding the lens membranes (aquaporins/Mip, Lim-2 or connexins) or the structural proteins of the cytosol of the lens fiber cells (the crystallins). Most forms of inherited cataracts are dominant, while recessive forms are less prevalent. However this may vary in different ethnic groups. Infantile cataracts are not necessarily progressive, thus may have little or no impact on adult visual acuity when affected embryonic lens nucleus become overlaid with layers of transparent fibers. Comprehensive characterization of infantile cataracts is given in recent reviews.(10),(12)
Studies on hereditary congenital cataracts have led to the identification of genes involved in the formation of these cataracts. Knowledge of the structure and function of a particular gene and the effect of disease-associated mutations on its function are providing insights into the mechanisms of cataract formation. Identification of the disease gene requires both the relevant clinical data as well as genetic data on the entire pedigree in which the disease is found to occur. Genes for hereditary cataract have been mapped by genetic linkage analysis -- one examines the inheritance pattern of DNA markers throughout the genome in all individuals of the pedigree and compares those with the inheritance of the disease. Co-segregation of a set of markers with disease implies that the disease gene is present at the same chromosomal location as those markers.
The genes so far identified for hereditary cataracts in both humans and animal models encode structural lens proteins, gap junction proteins, membrane proteins and regulatory proteins involved in lens development (Table 1). Knowledge of the mechanisms of hereditary cataract may also help us understand the manner in which environmental and nutritional factors act on the lens to promote opacification.
Environmental Cataracts
The etiology of environmental cataracts includes exposure to physical factors such as ionizing and infrared radiation,(13) chemical and mechanical injury, electric shock, etc., factors that challenge the integrity of the lens capsule, cells and their membranes and result in lens opacifications. Toxic or drug-induced cataracts are common complications following systemic treatment with corticosteroids, anticholinesterases, phenothiasines and radiomimetic drugs, as well as from products that increase the concentration of metal ions.(10) A separate class of risk factors that facilitates cataractogenesis includes exposure to parallel ocular pathologies like rubella (in children), glaucoma, uveitis, retinitis pigmentosa, ischemia, retinal detachment, intraocular tumors and metabolic diseases (e.g., diabetes),(14),(15),(16) as well as ethnicity.(17)
Age-Related Cataractogenesis and the Role of Oxidative Stress
Aging is, worldwide, the most significant environmental risk factor for cataract formation: [http://www.nei.nih.gov/health/cataract/cataract_facts.asp] A number of changes in the lens, the eye and the entire organism that are associated with ageing contribute to the development of age-related (senile) cataracts. Traditionally, aging is associated with increased oxidative stress, decreased anti-oxidative capacity and overall tolerance to stress. Indeed, lenses with age-related cataract are characterized by opacification and coloration in the nucleus, and by extensive protein oxidation.
Oxidation affects lens tissue homeostasis in several ways, impeding structural protein and membrane lipids stability,(7),(18),(19),(20),(21) crystalline solubility and chaperone activity,(22),(23),(24) intercellular transport,(6) and facilitating extensive post-translational modifications of vital lens proteins,(25),(26) as we have discussed earlier. Several studies focusing on these and other factors associated with ageing contributed to significant advances in understanding molecular causes of are-related cataractogeneses. Here we will briefly review these molecular causes.
Oxidation and Post-translational Modification of Proteins
Oxidation is accompanied by the loss of protein conformation (folding), which is essential for function. The extent of protein oxidation is typically assessed by the degree of oxidation of methionine residues and loss of sulfhydryl groups that are critical for proper protein folding. In advanced cataracts, >90% of protein sulfhydryl groups are lost and almost half of all the methionine residues in the nuclear proteins become oxidized.(6) Oxidation affects membrane lipids and facilitates post-translational modification (PTM) that are detrimental to solubility and folding of the lens proteins. Most common PTMs include isomerization, deamination, truncation, glycation, as well as binding of proteins to membranes.(7)
The key factor preventing oxidation seems to be the concentration of antioxidants, particularly nuclear glutathione (GSH).(5) Reduced gluathione (GSH) is a major antioxidant in human tissues that is essential for reduction (inactivation) of hydrogen peroxide and lipid hydroperoxides to water and the respective alcohol. During this process, GSH is oxidized to GSSG or the oxidized form of glutathione, which can then be enzymaticaly recycled back to the reduced form, GSH.
Provided that nuclear GSH levels can be maintained above 2 mM, it appears that significant protein oxidation and posttranslational modification by reactive small molecules such as ascorbate or UV filter degradation products are not observed. Glutathione in the lens is synthesized from its constituent amino acids and degraded by mechanisms involving transpeptidation and hydrolysis. The turnover of GSH in the lens results from its catabolism rather than the transport of GSSG, as is the case in red blood cells and some other tissues.
Three aspects of the functional role of GSH in cataract formation are considered. First, GSH may be important in maintaining protein thiols in the reduced state, thus preventing the formation of high molecular weight protein aggregates, which are the basis for light scattering and lens opacification. A second function may be to protect membrane -SH (sulfhydryl) groups that are important in cation transport and permeability. A third functional role is to detoxify hydrogen peroxide and other organoperoxides. The glutathione redox cycle is intimately involved in the detoxification of highly reactive H2O2, which is normally present in the aqueous humor.
Remarkably, research shows that in many cases there may be no significant oxidation of proteins in the center of the lens with advancing age, even past age 80.(6) The difference between these and cataractous lenses is in the level of nuclear GSH and other antioxidants which support lens transport. Adequate coupling of the metabolically-active cortex, the source of antioxidants such as GSH, to the quiescent nucleus is crucial, especially given that, it would appear, the cortex remains viable even in old lenses. Therefore, it is vital to understand the reason for the onset of the lens barrier (between the active superficial layers and the quiescent core of the lens).
The lens barrier, which becomes apparent in middle age, acts to impede the flow of small molecules between the cortex and the nucleus.(6),(7) The barrier may contribute to the lowered concentration of GSH in the lens nucleus after middle age. By extending the residence time within the lens center, the barrier also facilitates the decomposition of intrinsically unstable metabolites and may exacerbate the formation of H2O2 in the nucleus.
Types of Age-Related Cataracts
There are three major types of human age-related cataracts: nuclear, cortical and posterior subcapsular. Most common types are nuclear and cortical cataracts, which occur in the fully differentiated cells of the lens nucleus and cortex, respectively. A simple classification system of nuclear cataracts has been developed by Pirie(27) and it classes lenses into four groups based on the degree of nuclear color. Type I lenses are those that have been extracted primarily due to cortical cataract. Type II lenses (the first stage of nuclear cataract) are those which show an increase in nuclear color that is increased in Types III and more so in Type IV lenses (Figure 1). Despite the clear subjectivity of this system, it has produced a great deal of valuable biochemical correlates.

Figure 1. Human Age-Related Cataract Lenses Graded by Nuclear Color.

Graded according to the classification system developed by Pirie. By the permission of Elsevier Ltd.

Despite the clear subjectivity of this system, it has produced a great deal of valuable biochemical correlates.
Typical posterior subcapsular cataracts (PSCs) result from the accumulation of swollen cells at the posterior sutures, just beneath the posterior capsule. The morphology of PSCs suggests that they are caused by the abnormal differentiation, swelling and accumulation of malformed fiber cells at the posterior pole, where the light scattering occurs.(28) One proposed mechanism as to why these fibers fail to elongate is that the loss of their adhesive contacts with their neighbors and the lens capsule would mean that they would not reach sutures and would cause fibers to round up as they increased in volume, rather than elongate.
PSCs occur with lower frequency than nuclear or cortical cataracts in most populations (approximately 10% of cataract surgeries). There is an increased risk for developing PSCs in patients on steroid therapy and in individuals with hereditary retinal degenerations such as retinitis pigmentosa. Research in this area has been hampered by the lack of an animal model that develops PSCs resembling those seen in human patients.
According to localization in the lens and morphology, most common types of cataracts include anterior polar, posterior polar, subcapsular crystalline, lamellar, spoke, cortical, nuclear, punctate cerulean, coronary and total (opacity). By the type of opacities detected with slit lamp examination, lens opacities are classified as solid, pulverulent (blue dot) and crystalline. Additional sub-classes are often added in clinical practice to describe morphology of more exotic opacities: snowflake, snowball, floriform, spear, spirochaete-like, Christmas tree (Figure 2), etc. Sutural opacitfication adds aceuliform, stellate, myotonic, cerulean and are more typical for traumatic cataracts.(16)

Figure 2. Christmas Tree Cataract Phenotype.
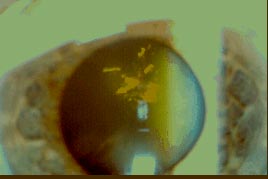
Courtesy of Victor Perez.

Fiber-based v. Non-Fiber-Based Cataracts
Classification by affected cell populations subdivides cataracts into fiber- and non-fiber-based cataracts. In the fiber-based group, the form of the cataract is determined by the arrangement of the lens fibers (Figure 3, A, B), while for non-fiber-based cataracts, form is unrelated to lens fiber organization.

Figure 3. Structural Organization of Mammalian Lens.
A. The bulk of the lens consists of concentric layers of fiber cells overlaid anteriorly by the epithelial sheath and encapsulated with thick basal membrane, the lens capsule. Progressive deposition of new fibers at the lens equator results in an onion-like architecture of the adult tissue shown schematically in B. Fiber cells retain full complement of their organelles only during elongation and maturation. After elongation is completed, these cells attach to the sutures and trigger ordered process of denucleation and organelle removal. Ultrastructural imaging shows details of fiber cell packing (C, top) and membrane interlocking (C, bottom), properties essential for tissue transparency and integrity during accommodation. (Courtesy of J. Kuszak)

Fiber-based cataracts are further divided into sutural and non-sutural, according to their relationship to the sutural system of the lens. The latter sub-group (see example in Figure 4J) is usually congenital by origin and familial.

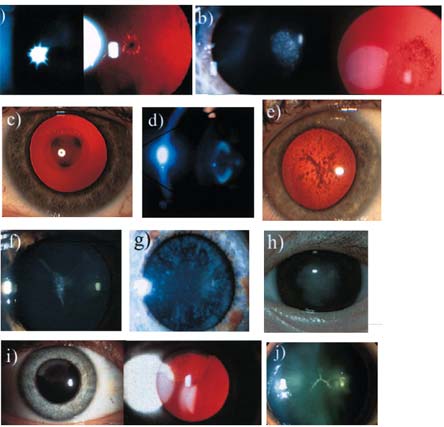
Figure 4. Cataract Phenotypes.
a. Anterior polar cataract. (Reprinted from Francis et al(26) with permission from the BMJ Publishing Group.) b. Posterior polar cataract. (Reprinted from Francis et al 26 with permission from the BMJ Publishing Group.) c. nuclear cataract. d. Lamellar cataract. (Reprinted from Ionides et al(*) with permission from the BMJ Publishing Group.) e. Pulverulent cataract. f. Aceuliform-like cataract. g. Cerulean cataract. h. Total cataract. i. Cortical cataract. j. Sutural cataract.

Typically, this opacity follows the line of the anterior or posterior Y suture and is, therefore, in the fetal nucleus, and much less frequently remains sub-capsular. This sub-type of cataract usually does not affect vision but, in the X-linked form, males have a significant cataract spreading beyond the suture. Some sutural cataracts, e.g., concussional, result from trauma affecting subcapsular fibers, where opacification is more obvious at the tips of the fibers.
Other types result from deposition of protein aggregates, metal and drug crystals at the vicinity of the lens sutures. Non-sutural cataracts affect particular lamellae of the lens both anteriorly and posteriorly. Most often they result from an insult affecting the outer shell of younger fibers for a limited time and is, thus, causing a shell-like opacity termed lamellar cataract (Figure 3D).
Non-fiber based cataracts vary significantly in shape and localization and include subcapsular (anterior and posterior, see Figures 3A, B), coronary, glaucoma flecks, Christmas tree (Figure 2), etc.
Secondary Cataracts
A common complication of cataract extraction surgery is the formation of secondary cataracts. Modern cataract surgery is performed by removing the lens fiber mass and implanting a clear plastic intraocular lens (IOL) into the remaining "capsular bag." Residual lens epithelial cells migrate under the IOL to the posterior capsule and form an opaque fibrotic layer, the secondary cataract. The plaques of fibrous tissue contain myofibroblast-like cells embedded in an extracellular matrix and scatter light.
The fibrous plaques found in secondary cataracts result from the transdifferentiation of epithelial cells into myofibroblast-like cells.(29),(30),(31) Significantly, lens epithelial cells can be experimentally stimulated to acquire many of the properties of myofibroblasts by treatment with the cytokine, TGFβ.(32) The absence of a "template" of preexisting fiber cells might preclude regular fiber cell morphogenesis and turn these cells into swollen shape elongated "entoids." Similar growth at the lens equator, in the vicinity of the haptics that position the IOL in the capsular bag, contributes to opacities called Soemmering's Ring.(33) Because it is positioned out of the optic axis, Soemmering's Ring does not degrade the visual image. Secondary cataracts develop in about 5-15% of patients who undergo successful cataract surgery and are commonly corrected by a laser treatment that clean away cellular plaques.
Perspectives of Cataract Prevention
Given the association between oxidative damage and age-related eye debilities, it is not surprising that over 70 studies have attempted to relate antioxidant intake to risk for two major age-related eye disorders, cataract and maculopathy. There are many researchers who believe that good nutrition and intense antioxidant supplementation can help reduce the risk of age-related cataract. The results of observational studies suggest that a healthy lifestyle with a diet containing foods rich in antioxidants, particularly lutein and zeaxanthin, as well as n-3 fatty acids, appears beneficial for AMD and possibly cataract.(34)
However, even the most potent anti-oxidants do not guarantee the beneficial effects on vision challenged by a cataract. Thus, on the basis of the evidence-based review, the FDA concluded that no credible evidence exists for a health claim about the intake of lutein or zeaxanthin (or both) and the risk of age-related macular degeneration or cataracts.(35) Another study concluded that a multivitamin-multimineral supplement with a combination of vitamin C, vitamin E, beta-carotene and zinc (with cupric oxide) is effective to reduce the risk of cancer,(36) is recommended for AMD but not cataract.(34) A healthy diet and basic preventive strategies (e.g., wearing sunglasses and a hat with a brim to block ultraviolet sunlight) can effectively delay cataracts. In addition, it should be mentioned that the efficacy of preventive measures might be quite different in industrial and the third world countries where Vitamin A deficiency and measles are still the major causes of severe visual impairment/blindness in children.(37)
Summary
Protection of the structural elements and homeostatic machinery of the lens from damage secondary to oxidation, toxicity and other deleterious factors, as well as correction of cataractogenic genetic defects, represent two major avenues of perspective cataract prevention and therapy. Meanwhile, antioxidant supplementation, cautious use of steroids and other drugs and improved eye surgery have the highest potential to lower the risk of age-related and secondary cataracts.
Acknowledgements
The author would like to acknowledge Victor Perez for help with cataract pictures, Julia Shestopalov for the help with figures.