Course Authors
Eli A. Friedman, M.D.
Dr. Friedman has received grant/research support from Alteon within the past three years.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
In this conference, I raise two issues of concern to all clinical nephrologists. First, interpretation of the commonly employed Serum Creatinine test versus the specialist's requested Creatinine Clearance. The surprising development is that the serum creatinine is not only acceptable, but may be preferred to the difficult-to-obtain creatinine clearance; second, the need to assess and quantify comorbid complications as a means of deciding when to do what for patients with progressive loss of kidney function.
Serum Creatinine versus Creatinine Clearance
All nephrologists appreciate the hyperbolic relationship between serum creatinine concentration and actual amount of renal function as judged by the glomerular filtration rate (GFR)as depicted in Figure 1.
Figure 1. Relationship Between Serum Creatinine and Endogenous Creatinine Clearance.
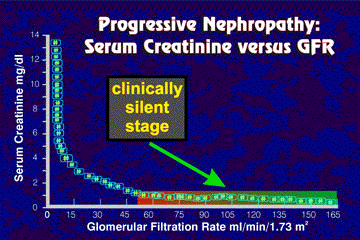
The curve drawn from several hundred measurements of serum creatinine and 24-h creatinine clearance shows the hyperbolic relationship between both variables. Note that more than one-half of renal reserve is lost before the serum creatinine rises above the normal range. Also evident is the increasingly greater rise in serum creatinine for each proportionate loss of creatinine clearance as renal failure becomes more severe.
While we would prefer a properly collected 24-h urine sample to calculate a creatinine clearance, we settle for the more practical and less expensive serum creatinine level. I have always felt a bit uneasy over choosing the easier path of a simple blood test in that accuracy was being sacrificed for expediency. Now, however, there is the happy finding in an initial report from the African-American Study of Kidney Disease and Hypertension (AASK) Pilot Study, that estimation of the GFR by serum creatinine values is both reasonable and reliable.(1)
My classification of this study as good news is based on multiple criticisms from editors who demanded radionuclide or inulin clearances as a condition for acceptance of manuscripts. Clinicians, will also cheer this report because it vindicates what they do out of necessity. As a prime example, transplant surgeons have followed for decades their allograft recipients with regular testing of the serum creatinine level to the dismay of their colleagues in nephrology who disparaged the results as unscientific or imprecise at best. AASK researchers compared GFR estimates by four methods: 100/serum creatinine, Cockcroft-Gault equation, creatinine clearance from a 24-h urine collection and a new regression equation derived from the pilot study data. As a reference standard, the renal clearance is measured by(1),(2),(5) I-iothalamate. In those evaluated, the mean serum creatinine was 1.67 Å 0.89 mg/dl, the 24-h creatinine clearance was 68.1 Å 27.6 ml/min/per 1.73 m,(2) the creatinine clearance calculated from the serum creatinine by the Cockcroft-Gault equation was 59.3 Å 21.7 ml/min/per 1.73 m,(2) and the actual GFR was 68.8 Å 26 ml/min/per 1.73 m.(2)
The investigation concluded that the Cockcroft-Gault formula underestimated GFR by nearly 10 ml/min/per 1.73 m;(2) this equation was computed from data collected in white men while the present study was restricted to African-Americans who have different dietary habits and greater muscle mass. While both 100/serum creatinine and serum creatinine values were useful for estimating GFR in African-Americans with long-standing hypertension, key findings of the study were that estimates of GFR relying on either 100/serum creatinine or the new equation were the most precise. Indeed, in 193 hypertensive African-American screenees (142 men, 51 women), the 24hr creatinine clearance was inferior to the serum creatinine as a surrogate for GFR estimation.
Admittedly, physiologically-oriented nephrologists will still insist on clearances. Clinicians, however, may breathe easier with the consoling support of a large study affirming reliance on simple serum creatinine measurements.
Co-Morbidity in Renal Insufficiency
Only rarely do patients under treatment for renal disorders die directly from either uremia or the direct effects of less advanced kidney disease. Prior to the availability of dialytic therapy, convulsions and coma, potassium intoxication, and bacterial and fungal infections were terminal events in the course of progressive kidney disease. In 1997, however, death attributed to kidney disease results from coincident dysfunction in extrarenal vital organ systems, especially the cardiovascular system.
Nephrologists providing comprehensive care to ESRD patients regularly devote more attention to these co-morbid risk factors than to delivery of dialysis or manipulation of transplant immunosuppression. Particularly in diabetic patients sustained by hemodialysis, management is decidedly more difficult because of co-morbid disorders than in an age and gender matched nondiabetic person. It is the toll of coincident extrarenal disease especially blindness, limb amputations, and cardiac disease that limits, or preempts rehabilitation, not accumulation of nitrogenous wastes or deranged electrolyte or fluid balance. For example, while provision of a hemodialysis vascular access in a nondiabetic patient is minor surgery, the same operative stress in a diabetic patient may prompt heart failure or life-threatening septicemia.
Co-Morbidity in Diabetic Nephropathy
Because diabetes tops the list of causes of ESRD and provokes the greatest co-morbidity during its treatment, awareness of the variety of co-morbid risks permits early corrective intervention.
Table 1. Co-Morbidity in Diabetic Nephropathy.
|
Listed in Table I are the major co-morbid concerns in the management of diabetic ESRD patients. Diabetic retinopathy ranks at the top with heart and lower limb disease as major concerns in overall patient care. More than 95 % of diabetic individuals who begin maintenance dialysis or receive a renal allograft have undergone laser treatment and/or vitrectomy for retinopathy. We routinely collaborate with an ophthalmologist skilled in retinal disorders. By this tact, laser and/or vitreous surgery can be integrated as a component of comprehensive management.(2) Similarly, we consult even in asymptomatic patients a cardiologist familiar with uremia in diabetic patients. Coronary angiography, (if indicated), is performed as a valuable maneuver to detect those for whom prophylactic coronary artery angioplasty or bypass surgery is likely to extend life. Included on our renal team is a podiatrist who delivers routine foot care. The podiatrist has by regular surveillance of patients at risk of major lower extremity disease sharply reduced the chance of amputations, a complication noted in about 20% who do not receive podiatric care.
Autonomic neuropathy expressed as gastropathy, cystopathy, and orthostatic hypotension is a frequently overlooked, highly prevalent disorder impeding life quality in the diabetic with ESRD. Diabetic cystopathy, though common, is frequently unrecognized and confused with worsening diabetic nephropathy and is sometimes interpreted as allograft rejection in diabetic kidney transplant recipients. In 22 diabetic patients who developed renal failure 14 men and eight women of mean age 38 years an air cystogram detected cystopathy in eight (36%) manifested as detrusor paralysis in one patient; severe malfunction in five patients (24%); and mild impairment in one patient. Gastroparesis afflicts one-quarter to one-half of azotemic diabetic persons when initially evaluated for renal disease.
Pregnancy in diabetics with proteinuria or azotemia, previously regarded as an unavoidable prelude to disaster in terms of fetal loss and/or maternal risk of death is now managed with a high probability of successful outcome. Miodovnik et al. followed 182 pregnant women with IDDM, 46 of whom had overt nephropathy for a minimum of 3 years after delivery and concluded that pregnancy neither increases the risk of subsequent nephropathy nor accelerates progression of preexisting renal disease.(6) In an equally encouraging series from Finland, Kaaja et al. followed 28 diabetic women for seven years after delivery compared with 17 nulliparous controls matched for age, duration of diabetes, and severity of vasculopathy and concluded that: "pregnancy does not seem to affect development or progression of diabetic nephropathy."(7)
The Co-Morbid Index
Longitudinal comparisons of ESRD patient outcomes including survival and benefit of treatment regimens require that we define equivalent cohorts at the initiation of observation and therapy. An inventory of the type and severity of common co-morbid problems is the essential step at the start and for subsequent inter group comparisons. By establishing a numerical ranking for the presence and severity of each extrarenal condition, a co-morbid index (Table 2) is created. Our empiric co- morbid index is reliable and reproducible by multiple investigators.
Table 2. Variables in Risk During Treatment of Renal Insufficiency, The Co-Morbidity Index.
|
To obtain a numerical Co-Morbidity Index for an individual patient, rate each variable from 0 to 3 (0 = absent, 1 = mild - of minor import to patient's life, 2 = moderate, 3 = severe). By proportional hazard analysis, the relative significance of each variable can be isolated from the other 12.
Cardiovascular Disease
Patients receiving renal replacement therapy experience a 16-fold to 19-fold increased risk of myocardial ischemia and infarction compared with age-matched populations without renal failure.(8) At very high risk are diabetic and younger nondiabetic patients. At least 50% of all deaths during ESRD management are the result of cardiovascular disease. This reality is apparent in Figure 2 drawn from the most recent report of the United States Renal Data System.(9)
Figure 2.
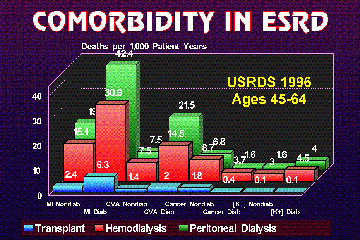
Extracted from the United States Renal Data System 1996 Annual Report, these three bar graphs show the major co-morbid risk factors ending in death for peritoneal dialysis, hemodialysis, and kidney transplant patients. For each ESRD therapy, diabetics have much higher death rates. Note the better survival for transplant recipients.
As shown in Figure 2, whether the ESRD patient is treated by hemodialysis, peritoneal dialysis, or kidney transplantation, the greatest risk of death in each treatment modality is heart disease. Predictors of near-term death have been identified.(10) The strongest predictor of myocardial infarction or sudden death was serum lipids on admission. In this prospective study of 196 diabetic patients started on hemodialysis at 28 German facilities. Maximal life-extension in ESRD is contingent on appropriate preventive medicine to forestall cardiovascular disease (see K. Lance Gould's Cyberounds® conference on lowering lipids.) Wheeler reviewed the multiple variables typically found in uremia threatening cardiac integrity including: heightened endothelial reactivity, enhanced platelet aggregation, increased leucocyte adhesion, proliferation of endothelial smooth muscle cells, impaired renal excretion of homocysteine accelerating atherosclerosis, and hypercalcemia.(11) Small series of dialysis patients show an inverse relationship between the number of co-morbid risk factors and survival.(12)
It follows that recognition and correction of co-morbid risk factors especially hyperlipidemia, hypertension, and coronary artery insufficiency will prolong the lives of patients with chronic renal disease.(13) Introduction of dobutamine stress echocardiography now permits sorting of patients about to undergo surgery identifying that subset most likely to die without preparatory coronary revascularization.(14)
Poor control of intravascular volume, arterial hypertension, hyperuricemia, and hyperlipidemia can be rectified with pharmacologic therapy, predialysis and increased delivered dialysis during ESRD.
Hypertension is a major confounding factor in the genesis and progression of nephropathy. In hypertensive subjects with NIDDM > 10 years, 36% had impaired renal function defined as a glomerular filtration rate < 80 ml/min/1.73 m2 or a serum creatinine concentration > 1.4 mg/dl and 75% had microalbuminuria or clinical proteinuria.(15) At every degree of renal insufficiency, normalization of hypertensive blood pressure will benefit survival. Hypertension occurs early during progression of renal disease and is present in four out of five patients who live to manifest ESRD.(16) Left ventricular hypertrophy, a step down the road to heart failure and death, afflicts more than half of all ESRD patients and probably is the consequence of hypertension and anemia.(17)
Dyslipidemia is noted in three-quarters of dialysis patients and as many as 80% of renal transplant recipients.(18) The means are now in hand to deal with anemia (erythropoietin), dyslipidemia (statins, gemfibrozil, fish oil), and hypertension (angiotensin converting enzyme inhibitors), thereby minimizing the liability imposed by these co-morbid risk factors (see the Cyberounds® conference on ACE inhibitors.)
Nephrology, as a specialty, holds the attraction of caring for the whole patient and not just an organ system. Newly emphasized recognition of co-morbid risk factors translates into a mandate for comprehensive patient management likely to be rewarded with both improved rehabilitation and extended life for individuals undergoing progressive loss of kidney function.