Course Authors
James R. Lupski, M.D., Ph.D.
In the last three years, Dr. Lupski has received grant/research support from WIH, MDA, FFB and Merck & Co., Inc. He has also served as a consultant for Athena Diagnostics.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Genetics is becoming a factor in the diagnosis and treatment of all kinds of disorders. While aware of medical genetics' growing importance, many clinicians feel it is difficult to keep pace with this rapidly advancing area. In his first Cyberounds® conference, Jim Lupski outlines the basic terrain of the genome project and introduces some of its issues and vocabulary. Dr. Lupski and the editors welcome your feedback and comments as we try to make this conference serve your needs as well as reporting on developments in the field.
-- The Editors
Introduction
Congenital abnormalities or birth defects are the leading cause of death among infants (0-364 days old) and by age 25 years eight percent of liveborns will be diagnosed with a disorder that has a major genetic component. The goals of the human genome project are to identify all genes in the human genome, determine their structure and function, and elucidate their involvement in human disease.(8) In this first Cyberounds® Medical Genetics conference, I will try to walk us through some of the basic concepts and terminology in this rapidly changing field of medicine.
Mutation and Human Genetic Disease
As mentioned, population-based studies in Canada on 1,169,873 live births demonstrate the substantial impact that genetics plays on human health. More than 79/1000 (~ 8%) of liveborns will suffer from a genetically based disorder by age 25.(1) This genetic load occurs in several ways:
- Single gene disorders account for 3.6/1000 (.36%)
autosomal dominant 1.4/1000 (.14%)
autosomal recessive 1.7/1000 (.17%)
X-linked recessive 0.5/1000 (.05%) - Chromosome abnormalities account for 1.8/1000 (.18%)
- Multifactorial disorders are believed to account for 46.4/1000 (4.64%)
- Genetic unknown=0.12%
- Congenital anomalies with a genetic etiology = 2.66%
(all congenital anomalies = 5.25%)
Types of Mutation
A mutation is any replicable change of DNA material which can occur at the level of the genome, chromosome or the gene itself. It is this change which is responsible for so-called genetic disorders. Not all mutations, however, produce disorders, as some remain unexpressed, without noticeable effect on the organism.
Genome Mutations
The genome is defined as the total genetic material contained within the chromosomes of an organism. Genome mutations occur at a frequency of about 10-4 to 10-5 and represent a portion of the genome that has changed. Polyploidy occurs when an extra copy of every one of the chromosomes is made, trisomy when only an extra copy of a single chromosome occurs. Trisomy 21 (Down syndrome) is perhaps the most frequently observed genetic disease, occurring in 1:660 newborns. Trisomy 18 (Edward syndrome -1/3000) occurs more frequently than trisomy 13 (Patau syndrome -1/5000). If an entire chromosome is absent, most frequently in Turner syndrome (45,X karyotype), the disorder is called a monosomy.
Chromosome Mutations
Structural rearrangements in chromosomes that can be detected microscopically by changes in the banding patterns produced by dye specific staining of chromosomes are said to be chromosomal mutations. There are several major types:
Microdeletions: deletion of a small portion of a chromosome, sometimes several contiguous genes, produces a set of syndromes which are now being appreciated to a much greater extent. Microdeletion syndromes will be the subject of a future Cyberounds®.
Microduplications: an extra exact copy of a specific region of a specific chromosome.
Inversions: a segment of chromosome being in an inverted order.
Translocations: a piece of a chromosome translocating to another chromosome. Individuals that harbor these translocations are said to be balanced (because they have the proper amount of genetic information) but their offspring can, as the result of malsegregation of chromosomes producing duplications and deletions, be unbalanced. It is estimated that 1 in every 550 individuals on this earth may contain a balanced translocation. The unbalanced conceptus products of a balanced translocation carrier can be clinically suspected in pedigrees with frequent miscarriages (hence the need to evaluate chromosomes when three or more miscarriages occur in a family).
Gene Mutations
Gene mutations occur in a frequency believed to be between 10-5 and 10-6. Different types of mutations exist:
DNA rearrangments
Deletions in portions of a gene may occur and usually a sequence motif, such as direct repeats of less than 20 basepairs, may be involved.
Duplications are exact copies of a DNA sequence. Interspersed repetitive sequences can be substrates for recombination leading to intragenic duplications
Substitutions involve replacement of one sequence motif with another sequence.
Insertions can occur by the movement of specific sequences and insertion into a gene. Examples include AluI repetitive sequence insertion leading to neurofibromatosis type I and the insertion of LINE element associated with hemophilia.
Figure 1. DNA Rearrangements.
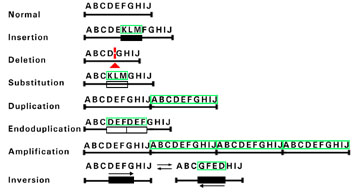
Mutations resulting from DNA rearrangements usually involve gross loss or rearrangement of many base pairs. The letters are used to depict several DNA bases. Below each set of letters are lined drawings of the DNA sequences. Each alteration in DNA sequence resulting from a particular rearrangement is surrounded by green lines. The triangle denotes a deletion.
Point Mutations
A point mutation is a change affecting a single position in a gene. For dominant and sex-linked recessive conditions, differences in incidence/prevalence are likely a reflection of underlying differences in the relative mutability of certain genes in the germ line. They are thought to arise by chemical (e.g., deamination of 5-methylcytosine or 5mC, depurination), physical (e.g., DNA slippage), or enzymatic (e.g., postreplicative mismatch repair of exonnucleolytic proofreading) mechanisms. The efficiency of these mechanisms is known to be sequence dependent and, therefore, these are nonrandom occurrences in the genome which are called mutation hot spots.
Transitions are changes of a purine base (adenine A or guanine G) to another purine (A-->G or G-->A) or of a pyramidine base (cytosine C or thymine T) to another pyrimidine (C-->T or T-->C). They occur much more frequently than transversions (purine change to pyrimidine or pyrimidine change to purine). In fact, transitions occur 14 times more frequently than transversions. Mutations can be silent and not change the coding of a specific amino acid, while missense mutations lead to a different amino acid substitution. Nonsense mutations are point mutations which change a triplet codon that encodes a specific amino acid. Frameshift mutations delete bases of greater than or less than three in number. This shifts the reading frame and produces a subsequent stop codon.
A substantial proportion of point mutations which have been found to cause human genetic disease occur as the result of missincorporation of bases during DNA replication. Most mutations that cause human disease are not caused by environmental agents or mutagens but actually represent the noise in the biology of the system.
CpG Dinucleotides
Of particular interest for point mutations is CpG dinucleotides. Point mutations in the human genome often occur at these CpG hot spots which are found in the methylated regions of the gene and it is there that the common transition changes (CG-->TG and CG-->CA) occur. These transitions occur via deamination and are responsible for many human diseases of genetic origin.
Detection of Mutation in the Human Genome
The mutations described above display a considerable range of base pairs that are involved. Therefore, the method with which to detect the mutation are different.
Chromosome 21, for example, represents 1.9% of the 3 x 109 base pairs that make up the human genome. This represents approximately 57 megabases, or 5.7 x 107 base pairs. Even if you were somehow able to sequence the entire chromosome 21 from a patient with Down syndrome you would not be able to observe this mutation. However, if you look at the entire genome at once by a chromosome karyotype you would see that there are three chromosome 21's in a patient with Down syndrome versus two copies of the chromosome 21 in normal individuals. It is important to note that there are no mutant genes in individuals with trisomy 21, but the phenotype represents the effects of gene dosage.
On the other hand, in a patient with sickle cell disease there is only one base pair change in the 3 x 109 base pair genome. You can look forever at a karyotype, but will never see any abnormal chromosomes. In order to find the genetic error you will have to sequence the DNA from the globin gene of the patient with sickle cell disease or look for a specific restriction site change (a change in DNA that alters a recognition site for an enzyme that will cut DNA at a specific sequence).
It can readily be seen that depending on the type of mutation very different methods are utilized to observe the alteration in the human genome (Figure 2).
Figure 2. DNA Size Ranges for Different Separation Methods.
| DNA Size | Enzyme Used | Gel Separation Method |
| 1 bp | DNA pol I | 12 % PAGE |
| 10 bp -100 bp | Restriction endonuclease with 4 bp recognition sites (4 bp cutters) | 8% PAGE |
| 100-10,000 bp | 6 bp cutters | 1% agarose gel electrophoresis |
| 1,000-100,000 bp | 6 bp cutters | 0.35% agarose gel electrophoresis |
| 10,000-10,000,000 bp | 8 bp cutters | PAGE |
| 10,000-10,000,000 bp | 8 bp cutters | Field Inversion Gels |
| 1,000,000-10,000,000 bp | -- | FISH |
| 10,000,000-100,000,000 bp | -- | High resolution chromosome banding |
| 100,000,000 bp | -- | Karyotyping |
| >1,000,000,000 bp | -- | Human genome |
Size ranges of DNA separation methods. On the left is shown the approximate size of range of the DNA molecules in base pairs (bp). The middle column shows t he enzyme used to generate fragments within the given size range on the left. The last column lists the method used to perform the separation.
The ability to apply the technique fluorescence in situ hybridization (FISH) has extended the capabilities of standard cytogenetics to observe submicroscopic duplication or deletions. FISH applies DNA probes labeled with a fluorescence marker and fluorescence microscopy to visualize these probes and thus extends the capabilities of conventional cytogenetics. However, the clinician needs to know where to direct the cytogenetics laboratory based on his or her clinical suspicion of a specific diagnosis. That is, a standard karyotype enables one to observe the entire genome at once in total but one can only resolve on the order of several million base pairs of sequence. A standard G-band karyotype enables one to count approximately 500 different stained bands in the clinical cytogenetics laboratory. If there are approximately an estimated 100,000 genes in the 3 x 109 base pair human genome, then standard cytogenetic techniques which would detect the missing or extra single band requir e at least 2-3 million base pairs of DNA (perhaps 100-200 genes) to be altered in order to visualize this. Microdeletions or microduplications can be better observed using fluorescence in situ hybridization but the region of the genome involved ne eds to be suspected so that those specific probes can be utilized.
Clinical Benefits of the Human Genome Project
It is estimated that the sequence of the human genome should be complete by approximately the year 2005. However, throughout the duration of the project, a substantial number of reagents have become available which are useful for disease gene mapping, diagnostics and predictive testing, as well as for investigating the pathophysiology of specific disease processes.
The availability of extensive genetic maps has increased the pace by which different disease genes are localized in the human genome and also enabled approaches to identify disease suceptibility loci for multigenic disorders such as diabetes, hypertension, and certain forms of cancer.
Novel mutational mechanisms, such as trinucleotide expansions, have immediately led to more precise diagnostic tools to establish a secure diagnosis, enable predictive testing, and enable the availability of prenatal diagnosis. The cloning of the human genes for rare biological molecules (human insulin, surfactant, erythropoiten, and in the future other chemokines, lymphokines, and neurotrophic factors) has led to the availability of critical reagents that may soon change the face of how we practice medicine.