Course Authors
Rebecca G. Wells, M.D.
Dr. Wells is Assistant Professor of Medicine (Gastroenterology), University of Pennsylvania School of Medicine, Philadelphia, PA.
Dr. Wells reports no conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss current thinking about the mechanisms of fibrosis
Discuss new modalities for diagnosing fibrosis
Discuss evidence supporting the possibility of fibrosis regression
Discuss new treatment options for fibrosis, and understand their relationship to concepts of regression.
Dr. Wells will discuss the unapproved use of Fibroscan® and MR elastography.
Liver fibrosis, like pathological fibrosis in other organs, has been termed "wound healing gone bad." Simply defined, it is the deposition of abnormal extracellular matrix (ECM) - scar tissue - in the chronically injured liver. The distribution of abnormal matrix can vary from periportal to pericentral. Similarly, the etiology of the chronic injury varies and can include chronic viral hepatitis (hepatitis B and C), alcohol abuse, non-alcoholic fatty liver disease (NAFLD), autoimmune disease, biliary obstruction, and congenital and metabolic anomalies. Nonetheless, fibrosis is a generic response: the underlying mechanisms are similar and the end result "architectural distortion with the formation of nodules, otherwise known as cirrhosis" is the same.
A fibrotic liver contains up to ten times as much total ECM as a normal liver. One of the first and most dramatic changes is the capillarization of the sinusoids, first described by Hans Popper in 1963, in which the sparse and loosely organized ECM of the normal sinusoids is replaced with an organized basement membrane. This is associated with profound changes in cell function, including dedifferentiation of hepatocytes and loss of fenestrations of the sinusoidal endothelial cells. Diffusion of soluble proteins and fluids between the sinusoidal blood and adjacent cells is impaired, and the synthetic and detoxification abilities of hepatocytes decrease. Later, as fibrosis progresses, dense bands of the rigid fibrillar collagens (collagens I and III) and other matrix molecules form. The mechanical changes and architectural distortion of the liver worsen, with the development of portal hypertension and the associated complications of end-stage liver disease.
Fibrosis and cirrhosis affect millions worldwide. In the United States alone, there are an estimated 400,000 people with cirrhosis, and the incidence will likely increase due to the epidemics of hepatitis C and NAFLD in this country. The incidence of deaths from end-stage liver disease due to alcohol abuse and hepatitis B infection remains high worldwide and there is yet no treatment for fibrosis or cirrhosis. Nevertheless, research in the past decade has shed new light on the mechanisms of fibrosis. New diagnostic tests and treatments are poised to enter the marketplace. The goal of this Cyberounds® is to introduce participants to new concepts in fibrosis that will enable them to better understand new tests and treatments in the future.
Mechanisms of Fibrosis
An Imbalance Between Matrix Synthesis and Degradation
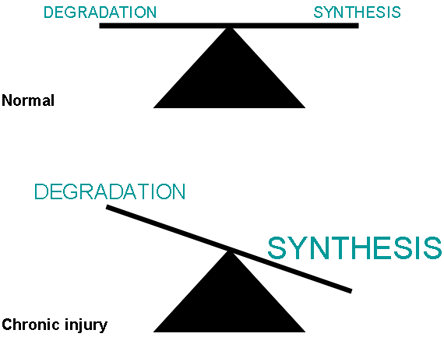
ECM is synthesized continuously even in the normal liver. In fibrosis there is a shift in the quality of the ECM as well as an increase in the amount of ECM deposited. While ECM synthesis is normally balanced by ECM proteolysis (breakdown), and overall levels of ECM remain constant, in the diseased liver there is an imbalance between synthesis and breakdown. Although matrix proteolysis may increase (enabling migration of fibrogenic cells), it is surpassed by an increase in matrix synthesis, leading to net matrix deposition, or fibrosis (Figure 1).

Figure 1. Fibrosis Results from an Imbalance Between Synthesis and Degradation.

The Myofibroblast in Fibrosis
The key pathogenic cells in fibrosis are myofibroblasts, cells that express the α-smooth muscle isoform of actin (α-SMA; Figure 2). These cells are fibrogenic, secreting excessive amounts of abnormal matrix, and contractile, contributing to the hemodynamic changes seen in liver fibrosis.(1) They are highly proliferative, secrete many profibrogenic growth factors and may be proinflammatory, all leading to self-perpetuation of the fibrotic process.

Figure 2. Myofibroblasts in Culture Stained with α-smooth Muscle Actin (Red).
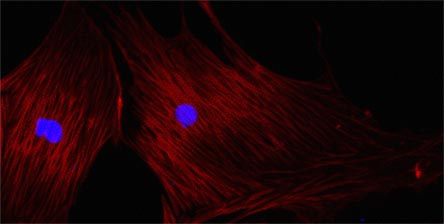
Note the actin arranged in stress fibers, which are contractile. Nuclei are stained blue.

Myofibroblasts in liver fibrosis originate from multiple precursor cells. The best known are hepatic stellate cells (HSC), the vitamin A-storing cells of the body, formerly called Ito cells or lipocytes. Emerging data suggest that other cells also undergo differentiation to myofibroblasts and contribute to fibrosis. These include cells from the bone marrow, hepatocytes, portal fibroblasts (PF) and biliary epithelial cells (BECs).(2),(3),(4),(5),(6) Because of their location in the portal tract, PFs and BECs may be particularly important in biliary (periportal) fibrosis. As compared to HSC, PFs and BECs differentiate in response to different stimuli and are, therefore, potentially attractive targets for disease-specific therapies.
Myofibroblast Differentiation
HSCs are the best-studied population of myofibroblast precursors. They "activate" and express α-smooth muscle actin when isolated and grown in culture, providing a model of myofibroblast differentiation in chronic liver disease (Figure 3).

Figure 3. Myofibroblastic Differentiation in Culture Mimics the Process in Liver Disease.
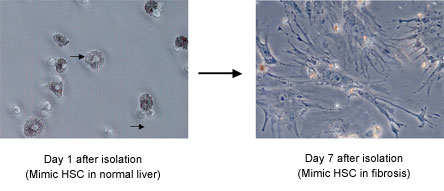
Arrows show vitamin A droplets in cells 1 day after isolation (left). Note lack of droplets in myfibroblastic HSC after 7 days in culture. Cells at day 7 are contractile and secrete large amounts of ECM.

Many factors that regulate HSC differentiation have been identified using this model system (Figure 4). These include cytokines and growth factors (especially transforming growth factor-β (TGF-β) and adipokines such as leptin), components of the renin-angiotensin system, extracellular matrix proteins, changes in matrix mechanics, reactive oxygen species and immune cells (including lymphocytes, neutrophils, Kupffer cells, and natural killer cells).(7) Some of these mediators have been validated in animal models of liver fibrosis and are targets for the development of anti-fibrotic therapies. Similar in vitro studies of other myofibroblast precursor cells, including PFs and BECs, are ongoing in many laboratories and may yield additional options for the development of disease-specific therapies.

Figure 4. Cellular, Soluble, and Mechanical Factors Leading to Liver Fibrosis.(7)
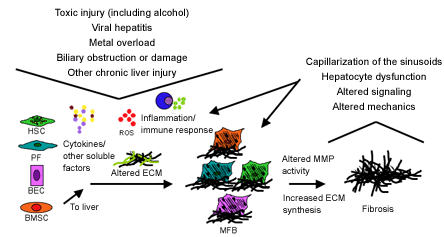
HSC: hepatic stellate cell; PF: portal fibroblast; BEC: biliary epithelial cells; BMSC: marrow stem cells; ROS: reactive oxygen species; MFB: myofibroblasts; MMP: matrix metalloproteinase.
Figure 4 was published in Drug Discovery Today: Disease Mechanisms, Wells RG., Mechanisms of liver fibrosis: New insights into an old problem, 3:489-494, Copyright Elsevier 2006.

Fibrosis is the generic response to chronic liver injury and many of the mediators noted above are part of this final common pathway. Distinct diseases, however, feed into the pathway in different ways. In chronic hepatitis C, viral-specific pro-inflammatory responses enhance fibrosis, while in alcoholic liver disease, pro-inflammatory and pro-fibrogenic alcohol metabolites have both direct and indirect effects on myofibroblast differentiation.(8) Oxidative stress plays a particularly important role in NAFLD. Additionally, many forms of liver disease act synergistically in causing fibrosis: both NAFLD and alcohol abuse, for example, accelerate the course of fibrosis in chronic hepatitis C.
Diagnosis of Fibrosis
The diagnosis of end-stage fibrosis (decompensated cirrhosis) is usually straightforward clinically. The presence of dermatologic stigmata, ascites, varices, encephalopathy or the hepatorenal syndrome, combined with laboratory evidence of profound synthetic dysfunction (elevated prothrombin time, low platelets and low albumin) clearly indicates cirrhosis. What is more difficult -- yet equally important -- is identifying the presence of lesser degrees of fibrosis and cirrhosis.
The amount of fibrosis informs many clinical decisions. It may determine whether or not a patient with chronic hepatitis C receives antiviral therapy and is used to counsel patients on the need for transplantation. In the transplant setting, particularly in patients with recurrent hepatitis C who are at risk for particularly rapid fibrosis, the degree and rate of progression of fibrosis is important in determining treatment of the underlying disease and immunosuppression.(9) As new antifibrotics become available in clinical trials and for general use, serial changes in fibrosis will be a key measure of efficacy.
Liver Biopsy
The liver biopsy is the gold standard for the diagnosis of fibrosis. Study after study, however, has shown it to be a poor standard, with cost, accuracy and the potential for life-threatening complications reducing its attractiveness. Transcutaneous biopsies, the most commonly used in the outpatient setting, are invasive, with significant morbidity commonly reported in the 1-5% range and mortality at 0.01 to 0.1%. Accuracy is low: even in diseases such as viral hepatitis that affect the parenchyma uniformly, sampling error is a problem, and cirrhosis is missed in up to 1/3 of patients. As a result, biopsies lack the precision required to determine the progression (or regression) of fibrosis over time.
Pathological analysis of biopsy specimens is also imperfect although the adoption of standard systems (including the METAVIR and Ishak systems(10),(11) for staging (by degree of fibrosis) and grading (by degree of inflammation) has improved interobserver reproducibility [for review, (12)].

Table 1. Stage of fibrosis.
| F0 | No fibrosis |
| F1 | Stellate enlargement of portal tract without septa |
| F2 | Stellate enlargement of portal tract with few septa (at least one on biopsy) |
| F3 | Septal fibrosis without cirrhosis |
| F4 | Cirrhosis |
Grade ranges from A0 to A3 based on periportal and lobular necrosis but not on portal inflammation.

Table 2. Ishak Scoring System for Fibrosis.
| 0 | No fibrosis |
| 1 | Fibrous expansion of some portal areas, with or without short fibrous septa |
| 2 | Fibrous expansion of most portal areas, with or without short fibrous septa |
| 3 | Fibrous expansion of most portal areas with occasional portal to portal bridging |
| 4 | Fibrous expansion of portal areas with marked bridging (portal to portal as well as portal to central) |
| 5 | Marked bridging (portal to portal and/or portal to central) with occasional nodules (incomplete cirrhosis) |
| 6 | Cirrhosis, probable or definite |
Grading provides a score of 0-4 for each of four parameters of necroinflammation: periportal or periseptal interface hepatitis (piecemeal necrosis); confluent necrosis; focal (spotty) lytic necrosis, apoptosis and focal inflammation; and portal inflammation.

The systems remain imperfect; for example, patients with minimal fibrosis (F0) may have subtle yet clinically relevant changes not detected by existing analyses. Additionally, these scoring systems are based on architectural changes in the liver, rather than the quantity of fibrotic tissue deposited. Recent data suggest that morphometric analysis to quantify fibrosis may provide an advantage over standard scoring systems.(13)
In the future, new methods to analyze biopsy specimens including stains for the myofibroblast marker, α-SMA, and the pro-fibrogenic growth factors, TGF-β and connective tissue growth factor, may overcome sampling error and improve accuracy, although they remain research tools. Similarly, the utility of measuring specific ECM components [freshly deposited matrix on the one hand and highly cross-linked (and, therefore, protease-resistant matrix) on the other hand] is under investigation.
Serological Tests
The limitations of the liver biopsy have led to the development of many serological tests which offer the possibility of being relatively non-invasive, inexpensive and quantitative. Some, such as the transaminases, prothrombin time and platelet level, are commonly used measures of liver dysfunction that are employed as surrogates for fibrosis. Others, including matrix proteases, matrix protease inhibitors (TIMPs) and matrix components [including hyaluronic acid (HA), laminin and the N-terminal propeptide of type III collagen (PIIINP)], have a more direct relationship to fibrosis. Unfortunately, all of the tested single marker tests have proved to be disappointingly nonspecific.
Panels of serum markers, combined in complicated and often proprietary algorithms to form single scores, offer more promise, although none has high enough sensitivity and specificity yet to replace the liver biopsy [for an exhaustive review, see (14)]. Generalizability studies and longitudinal validation are still lacking, and no test has been evaluated for its efficacy in patient management or predicting outcomes. Panels include the AST to platelet ratio index (APRI), the FIBROSpectII, and the European Liver Fibrosis Group (ELF) algorithm. FibroTest®/Actitest®, for use in patients with hepatitis C, is based on a combination of six serum markers as well as age and gender, and is so far the only algorithm to attempt to predict fibrosis on a continuum. In the long term, it may therefore prove the most useful.
New serological tests are under development, some including combinations of the markers discussed above. Direct indicators of ECM synthesis and turnover are being tested in new algorithms. New technologies, including proteomics, microarray analysis and glycomics (the study of altered patterns of glycosylation) offer the possibility of identifying new individual markers or patterns of markers that correlate with the stage of fibrosis.
Radiologic Tests
Radiologic tests, like serologic tests, offer the possibility of serial non-invasive measurements of fibrosis. Standard imaging, including ultrasound, CT scan and MRI, is used primarily to diagnose cirrhosis in compensated cirrhotics and is not useful either for determining the stage of fibrosis or for monitoring its progression in individual patients. Two new methods incorporating standard techniques, however, may well replace liver biopsy in the future.
Transient elastography (Fibroscan®), a method to estimate liver stiffness, has been assessed in multiple populations. Based on the hypothesis that fibrosis is linearly related to stiffness, this technique has shown promise in differentiating early from late fibrosis (especially when combined with Fibrotest®), although it has not performed well making finer distinctions. The Fibroscan® device is used increasingly in Europe, but is not yet available in the U.S. and has several limitations, including poor performance in obese patients and those with ascites [for review, see (15)]. A second modality based on similar concepts, MR elastography, combines sound wave and MR technology to determine liver stiffness. Early reports suggest that MR elastography may be able to distinguish accurately between different fibrosis stages, although it has not been validated beyond pilot experiments.(16),(17)
(Fibroscan® and MR elastography are not yet FDA-approved. Fibrotest®/Actitest® is FDA-approved and marketed in the U.S. as HCV Fibrosure®.)
Fibrosis Regression
Liver fibrosis was once believed to be a relentlessly progressive and permanent process, with cirrhosis and end-stage liver disease almost inevitable. It is now clear that this is not true, and that fibrosis can improve significantly if the underlying insult is removed. The first suggestion that this was the case in humans came from pioneering work by Ian Wanless which documented pathological features of biopsy specimens.(18) Since then, an analysis of ten trials covering more than 3,000 patients with hepatitis C concluded that antiviral therapy had been clearly demonstrated to reduce the rate of fibrosis progression.(19) Treatment studies of fibrosis resulting from other diseases (including hemochromatosis, Wilson's disease, autoimmune liver disease and biliary obstruction), have also shown that fibrosis is not permanent [for review, see (20)].
Regression is a more accurate term than resolution in fibrosis because matrix proteolysis and remodeling, while potentially dramatic, are unlikely to result in a normal liver.(21) Importantly, complete return to normal histology is unnecessary; significant functional improvements may occur with even modest changes in liver architecture. Regression of fibrosis requires both loss of myofibroblasts (primarily by apoptosis) and degradation of scar tissue deposited in the liver. There is considerable interest in understanding the factors that make scar tissue more or less susceptible to proteolysis, and in determining whether there is a "point of no return", when matrix breakdown becomes impossible. Some investigators have suggested that increased cross-linking of the collagenous scar tissue makes it increasingly protease resistant.(22) Cellularity and topography of the scar as well as the duration of disease may influence the degree of reversibility. Significant ongoing research is focused on understanding the histological and patient features that predict the possibility of regression. Whether or not all fibrosis and cirrhosis is ultimately reversible (and if the patient can be supported through its complications) is not yet clear.
Treatment of Fibrosis
Treating the Underlying Disease
Regardless of the etiology of fibrosis, the concept of regression has profound implications for treatment. It is now absolutely clear that removal of the underlying insult -- whether with antiviral medications, alcohol cessation or immunosuppressives -- is a key component of therapy [for review, see (20)]. As noted at the beginning of this Cyberounds®, matrix proteolysis occurs even during ongoing fibrosis. Fibrosis is the result of an imbalance between matrix deposition and degradation (Figure 1); thus, small changes on either side of the equation offer hope of partial regression. For this reason, it is particularly important to avoid anything that might exacerbate a chronic injury -- for example, drinking alcohol in the setting of chronic viral hepatitis.
New Therapies Against Fibrosis
There is still no magic bullet for the treatment of fibrosis. The ideal antifibrotic would both prevent new matrix deposition and induce matrix proteolysis and architectural remodeling (Figure 5). Many such drugs have been identified in animal models; however, data supporting their use in humans are sadly lacking. This may soon change, as there are a number of agents in clinical trials, some of which were developed based on an understanding of the pathogenesis of fibrosis.(23),(24)

Figure 5. The Dual Goals of Antifibrotic Therapy

Some new antifibrotics seek to prevent myofibroblast differentiation. Initial hopes focused on anti-inflammatory drugs such as vitamin E. Unfortunately, large clinical trials have not demonstrated these agents to be effective except in limited situations (autoimmune liver disease and severe alcoholic hepatitis). Interferon-γ, although also promising in early studies, did not cause fibrosis regression in a large prospective trial.(25) Growth factor inhibition, particularly with the potent profibrogenic agent TGF-β, is highly effective in animal models of fibrosis but has not been tested in humans and carries theoretical concerns about the long-term risk of cancer. Synthetic PPAR-γ ligands, including the hiazolidinediones, inhibit HSC activation in culture and are the subject of ongoing clinical trials, particularly in patients with NAFLD. The renin-angiotensin system is also a highly attractive target. It acts in part through TGF-β, and use of the angiotensin-1 receptor antagonist losartan has proven effective at reducing fibrosis in a pilot study of patients with hepatitis C.(26) Complementary and alternative therapies, although widely used, especially in Asia, thus far lack definitive evidence of efficacy.(27)
A second category of antifibrotics includes agents that treat existing fibrosis by enhancing matrix breakdown and inducing myofibroblast apoptosis. Although drugs that antagonize protease inhibitors (TIMPs) and enhance protease activity are promising in animal models, there are no human studies. Similar conclusions apply to pro-apoptotic agents, in particular gliotoxin, a fungal toxin that enhances HSC apoptosis.
Summary
In the past two decades, our understanding of fibrosis has increased dramatically. The variability of fibrogenic cells, the role of matrix proteolysis and the potential for fibrosis regression are new concepts that have revolutionized research in the field. In the next decade, these discoveries will drive changes in diagnosis and treatment. New diagnostic tests and treatments for liver fibrosis, some under investigation and some which will be discovered on the basis of new technologies, offer hope to patients with a disease that was, until recently, considered progressive and unstoppable.