Course Authors
Sunil Babu, M.D., Michael Liu, M.D., and Robert G. Lerner, M.D.
Dr. Babu is Fellow in Hematology/Oncology at New York Medical College/Westchester Medical Center, Valhalla, NY, and Dr. Liu is Resident in Internal Medicine at Cabrini Medical Center, New York, NY.
Within the past 12 months, Dr.Babu and Dr. Liu report no commercial conflicts of interest. Dr. Lerner reports research support from Boehringer-Ingelheim, Sanofi-Aventis and Bayer and is on the Speakers Bureau for Sanofi-Aventis, GlaxoSmithKline and Eisai.
Albert Einstein College of Medicine, CCME staff and interMDnet staff have nothing to disclose.
This activity is made possible by an unrestricted educational grant from ![]()
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the common side effects associated with EGFR inhibition
Discuss the correlation between dermatologic side effects of EGFR inhibition and response to therapy
Discuss the possible mechanisms of dermatologic side effects of EGFR inhibitor therapy
Discuss the common principles of management of dermatologic and non-dermatologic side effects.
Epidermal growth factor receptor (EGFR) inhibition is a novel approach in the treatment of colon, head and neck, pancreatic and non-small cell lung cancers. Although the most common toxicities of this category of agents are dermatological, other adverse effects such as infusion reactions, respiratory problems and electrolyte abnormalities can occur. It is important to understand the pathological basis of the side effects in order to be able to devise strategies to counter them. In this Cyberounds®, we review the current knowledge on the common toxicities associated with approved EGFR inhibitors.
Dermatologic Adverse Events
The severity of EGFR-related dermatological adverse events is graded using the National Cancer Institute Common Toxicity Criteria for Adverse Events (NCI CTCAE) (Table 1).
EGFR is expressed widely in human tissue including the epidermis, sweat gland apparatus and the hair follicle epithelium.(1),(2),(3) The cutaneous reactions to EGFR inhibitors include follicular eruptions, generalized xerosis, pruritis, hyperpigmentation, panniculitis, paronychia, alopecia, trichomegaly, fine brittle hair, ocular irritation and vaginal dryness (Table 2).
Current data suggest that skin eruption in patients taking EGFR may be a potential marker of response to therapy.(4),(5),(6),(7),(8) The degree of rash also appears to correlate with tumor responsiveness.(9),(10) A dose escalation randomized study using up to twice the standard dose of cetuximab in patients with metastatic colorectal cancer (mCRC) with nil or slight skin reactions on standard dose cetuximab treatment (EVEREST study) is ongoing.(11) Histological data from skin biopsy specimens indicate that the skin manifestations are similar with all the currently FDA-approved agents in the United States: erlotinib, gefitinib (restricted indication), cetuximab and the recently approved panitumumab, suggesting that this is a class effect of HER1/EGFR-targeted agents.
While erlotinib and gefitinib are oral tyrosine kinase inhibitors, cetuximab and panitumumab are monoclonal antibodies competitively inhibiting the binding of ligands for EGFR receptor. The epidermal growth factor receptor family (EGFR/ HER1/ ErbB1) is part of a complex signal transduction network that is central to several critical cellular processes. EGFR is a transmembrane glycoprotein of the subfamily that includes HER 2, 3 and 4 subtypes.(12),(13) The dermatological manifestations seen with EGFR inhibition are not observed with targeted inhibitors of HER2 receptor such as transtuzumab.
The term "acneiform eruption" is related to the usual distribution and appearance of the drug rash associated with EGFR inhibitors (Figure 1).(2),(14),(15),(16),(17),(18),(19) In contrast to acne, no black heads or whiteheads precede the follicular papules or pustules of EGFR-related acneiform eruption, which typically start with the formation of comedo by follicular hypercornification from seborrhea.(14),(18),(19),(20),(21) The distribution commonly involves scalp, face, chest, back, but usually spares the palms, soles and mucous membranes, although stomatitis and ocular manifestations can occur. Unlike acne, the EGFR inhibitor-induced rash can also affect areas such as the lower legs and dorsal arms.

Figure 1. Acneiform Eruption.
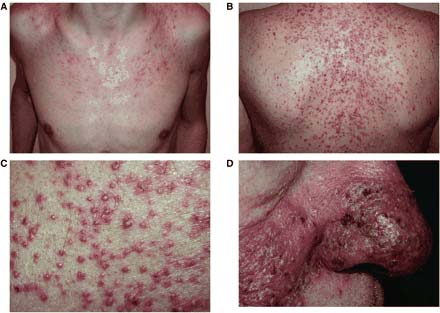
A. Papular lesions on the chest, B. V-shaped papulopustular eruption on the back, C. Close up of follicular pustules, D. Confluent pustules on the nose.(21)
Copyright © 2001, Published on behalf of the British Association of Dermatologists. Reproduced by permission.

Pathologic Findings
The mechanism by which EGFR inhibition leads to dermatological side effects is largely unknown. The lack of a reliable animal model has hampered the determination of the pathophysiology of EGFR inhibition-induced follicular rash. Epidermal growth factor (EGF) is a potent mitogen on cultured keratinocytes and leads to epidermal hyperplasia and hyperkeratosis.(3),(31),(32),(33),(34),(35) In the skin, EGFR is primarily expressed in undifferentiated proliferating keratinocytes in the basal and suprabasal layers of the epidermis and the outer layers of the hair follicle.
The earliest findings of EGFR inhibition include an infiltrate of T lymphocytes surrounding the follicular infundibulum. After one week of treatment with an EGFR inhibitor, the pathology usually shows superficial perifolliculitis involving hyperkeratotic and ectatic follicular infundibular suppurative inflammation or focal intraepidermal acantholysis with sparse neutrophilic infiltrate. The mechanism of this pattern of acantholysis has not been explained.(2),(9),(12),(14)
Among potential candidates of proteins involved in post receptor effects of EGFR inhibitors, the growth regulator p27kip 1 merits attention (Figure 2).(2),(12),(14),(36) It is a negative growth regulator that binds to and inactivates CDK-2 (cyclin-dependent kinase-2), thereby leading to cell cycle arrest in G1. EGFR inhibitors are known to increase the p27kip 1 expression in the keratinocytes.

Figure 2. Immunoreactivity for p27Kip 1 in Epidermal Keratinocytes.
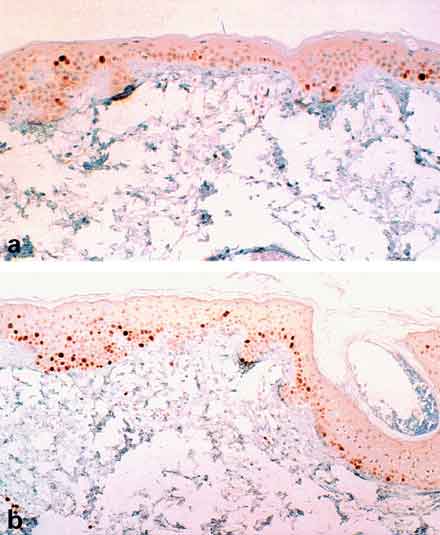
Fewer nuclei are immunopositive prior to treatment with cetuximab (a) than one week after treatment (b).(2)
Copyright © 2001, Published on behalf of the British Association of Dermatologists. Reproduced by permission.

It is also postulated that EGFR targeted therapies may affect the immune system by unblocking cutaneous chemokine production, which leads to leukocyte chemotaxis and infiltration of the skin. One of the postulated mechanisms for cetuximab-induced acne is excessive follicular hyperkeratosis which causes follicular plugging and ostial obstruction. This blockage can lead to rupture of the follicle wall, followed by chemotaxis of inflammatory cells and release of cytokines.(2),(14),(16)
The thickness of the stratum corneum layer is uniformly decreased in patients who were treated with EGFR inhibitors. Dryness and skin fragility can be reproduced in EGFR depleted mice, thus establishing the role of EGF in the maintenance of the integrity of the epidermal permeability barrier.
Hair growth abnormalities related to EGFR inhibition depend on the type and the location of the hair. In the scalp, the growth abnormalities manifest as alopecia, while the facial manifestations include hypertrichosis and trichomegaly. The normal hair cycle consists of three stages: anagen or the growth phase, catagen or the regression phase and telogen or the resting phase. It is thought that EGFR activation is required for progression from anagen to catagen. EGFR nude mice had hair follicles that were prevented from entering the catagen phase and thus produced abnormal hair growth. The hair changes reflect the role of EGFR and its ligands in hair follicle biology and hair cycling.(1),(21)
Periungual inflammation (paronychia) occurs later than the follicular rash. Histological examination shows a diffuse inflammatory infiltrate consisting of plasma cells, lymphocytes and some neutrophils within an edematous stroma. Evidence of an infectious cause (fungal or bacteria) has not been found at the onset of these lesions. Staphylococcus aureus has been cultured from some lesions but the pathologic significance is unknown.(2),(14),(21) EGFR inhibition also leads to a brittle, slower growing nail plate. Discontinuation of therapy results in consistent improvement of all the dermatological manifestations. There is also evidence to suggest that with continuation of treatment the rash dissipates or even resolves in most patients, though it persists in some. The mechanisms behind these differences are poorly understood but it is believed that pharmacogenetic and pharmacodynamic differences may play a role.(37)
In the ongoing EVEREST study, preliminary results suggest that immunohistochemistry (IHC) analyses, including EGFR, p-EGFR, Akt, p-Akt, ERK and p-ERK completed on 35 paired, pre- and post-treatment skin biopsies, demonstrate no strong correlations with toxicity. It has been shown that EGFR genetic polymorphisms in intron 1 correlate with response to the EGFR inhibitors in cell lines, although clinical validation is required. Perea et al. reported that cell lines with short number of CA dinucleotide repeats had a higher incidence of rash when treated with an EGFR inhibitor. These findings contrast with the lack of correlation found in clinical trials between expression of EGFR and activity of EGFR inhibitors. This contradiction may exist because of the different techniques employed to determine EGFR expression -- immunohistochemistry in studies finding lack of correlation and RT-PCR measurement of mRNA in studies showing correlation.(11),(37),(38)

Table 3. Anti-EGFR Therapy: Recommended Doses.
|
(30),(39),(40),(41)

Management of Skin Toxicity
Currently, since EGFR inhibition strategy is predominantly used in advanced stages of various malignancies, clinicians should diligently try to reduce the morbidity of the dermatologic side effects. Before patients begin treatment, they should be informed of the possible side effects so that apprehension about therapy at the onset of dermatological side effects can be minimized. This will also ensure patient compliance to the regimen as well as treatment of the skin manifestations only when required. It is also helpful to inform patients about the correlation of onset and intensity of the rash with response to therapy. Available data on medical management of the dermatologic adverse events remain scarce and a multidisciplinary approach with an experienced dermatologist is advisable especially in severe cases.(2),(14),(21),(42)
It is important that any strategy to overcome dermatological side effects should not interfere with the antitumor effects of EGFR inhibitors. For severe follicular rash, dose reduction or dose modification (divided doses) of the EGFR inhibitor is advised and in case of inadequate response discontinuation of the drug is recommended. Careful reintroduction can be attempted after the side effects subside. As different EGFR inhibitors are approved for similar indications, alternate agents may be tried, although the class effect may make it difficult to switch agents. It is also helpful to explain to the patients that the rash may dissipate with the continuation of the drug in many cases, although no predictive factors have been defined.
Topical agents that can cause skin irritation should be used with caution, as the skin of patients with EGFR-induced rash is abnormally sensitive. This hypersensitivity might explain the poor tolerance of some patients to topical agents such as retinoids. A promising strategy is the use of Vitamin K3 (menadione) as a potential therapy for the rash. Treatment with this agent restored the EGFR activity in mice pretreated with EGFR inhibitor. An interesting observation is that the EGFR inhibitor-induced rash spares the skin over a previously irradiated site.(43)

Table 4. Management Options for EGFR Inhibitor-induced Dermatologic Toxicities.
General measures
Pharmacologic Options Depending on the grade of the skin manifestation, different agents have been tried. No strong data support the use of any of the agents: Benzoyl peroxide, retinoids, tetracycline, minocycline, clindamycin, erythromycin, topical or oral steroids Immunomodulators such as tacrolimus, pimecrolimus, topical antibiotics |
(2),(9),(14),(21)

Table 5. Inhibitor Dose Modification Guidelines for the Management of Dermatological Toxicities.
| Severe acneiform rash | Suggested Dose Management |
|---|---|
| Cetuximab | |
| 1st occurrence | Delay 1 to 2 wk If improved, then continue at 250 mg/m2 If no improvement, then discontinue cetuximab |
| 2nd occurrence | Delay 1 to 2 wk If improved, then reduce to 200 mg/m2 If no improvement, then discontinue cetuximab |
| 3rd occurrence | Delay 1 to 2 wk If improved, then reduce to 150 mg/m2 If no improvement, then discontinue cetuximab |
| 4th occurrence | Discontinue cetuximab |
| Erlotinib | |
| Occurrence | Reduce dose by 50 mg decrement If improved, then continue at 100 mg PO daily. If no improvement, then temporary interrupt or discontinue erlotinib. |
| Gefitinib | |
| Occurrence | Delay 1 to 2 wk If improved, then restart at initial dose, 250 mg tab. If no improvement, then discontinue gefitinib |
| Panitumumab | |
| Occurrence | Hold if considered intolerable or grade 3 or higher If does not improve to grade 2 or less within 1 month, permanently discontinue. If improved to grade 2 or less after holding no more than 2 doses, resume at 50% of the original dose. If toxicities recur, permanently discontinue. If toxicities do not recur, subsequent doses may be increased by increments of 25% of the original dose until the recommended dose of 6 mg/kg is reached. |
(30),(39),(40),(41)

Although not dose limiting, conjunctivitis and keratoconjunctivitis sicca have been attributed to these agents. Sometimes trichomegaly (excessive eyelash growth) can happen and can cause corneal irritation or abrasion. During follow up visits patients should be evaluated for this condition and referred to an ophthalmologist if required. If the eyelashes are long they may need trimming to prevent corneal injury.(39),(40),
Non-dermatologic Adverse Events Associated with Anti-EGFR Therapy
Although dermatologic toxicities have received most of the attention since the introduction of these agents, largely the result of their relatively high prevalence, as well as their possible correlation with the response to therapy, it is important to be aware of some other side effects, which can be fatal if not recognized early. The non-dermatologic side effects vary from the non-specific side effects such as fatigue, headache, nausea, diarrhea, etc., to more unique adverse effects such as severe infusion reactions, hypomagnesemia and interstitial lung disease (See Table 6).
Intravenous administration of cetuximab, a human/mouse chimeric monoclonal antibody, may be associated with severe infusion reactions including bronchospasm, anaphylactic shock or cardiac arrest in approximately 3% of patients, rarely with fatal outcome (<1 in 1000). Approximately 90% of severe infusion reactions were associated with the first infusion of cetuximab, despite the use of prophylactic antihistamines. A one-hour observation period is recommended following the cetuximab infusion and longer for patients who experience milder reactions. Mild to moderate infusion reactions (chills, fever, dyspnea) are managed by slowing the infusion rate and administering antihistamines. Recently approved panitumumab, which is a recombinant, human IgG2 kappa monoclonal antibody, has a reported incidence of about 1% severe infusion reactions with intravenous administration. Fatal infusion reactions have not been reported with panitumumab. The difference in the incidence rate of infusion reactions between cetuximab and panitumumab may result from the murine component in cetuximab.(30),(41),(42)
Cardiopulmonary arrest or sudden death occurred in 2% (4/208) of patients with squamous cell carcinoma of the head and neck treated with radiation therapy and cetuximab, compared to none of 212 patients treated with radiation treatment alone. Although the etiology of these events is uncertain, close monitoring of serum electrolytes, including serum magnesium, potassium and calcium, during and after cetuximab treatment is recommended.(30)
Interstitial lung disease (ILD)-like events with rare fatalities have been reported with these agents. ILD-like events include interstitial pneumonia, interstitial lung disease, obliterative broncholitis, pulmonary fibrosis, acute respiratory distress syndrome and lung infiltration. Determining the actual incidence of anti-EGFR therapy-related ILD-like events is complicated by factors such as the underlying neoplastic disease, adverse events caused by other chemotherapeutic agents, growth factors, opportunistic infections, as well as concomitant radiation therapy. The reported incidence of ILD in treatment with gefitinib for NSCLC was about 2% in the Japanese post-marketing experience and about 0.3% in approximately 23,000 patients treated with gefitinib in a U.S. expanded access program. Smaller studies conducted in Asia have shown greater incidence of ILD-like events. The difference in the rates may be secondary to ethnicity related factors.(39),(40),(44),(45)
Patients often present with acute onset of dyspnea, sometimes associated with cough and low-grade fever, which often becomes severe within a short time and requires hospitalization. At the first sign of onset of respiratory related events, the drug should be discontinued and thoroughly investigated. Treatment should be discontinued if ILD is confirmed. Steroid therapy should be initiated if ILD is suspected and symptoms may gradually resolve after discontinuation of EGFR therapy. Since patients with pre-existing pulmonary fibrosis and other pulmonary comorbidities have a higher risk of developing this adverse effect, these patients should be monitored closely, especially during the early phase of anti-EGFR therapy.
NCI-CTC grades 3 and 4 incidences of hypomagnesemia have been noted with the use of anti-EGFR monoclonal antibodies cetuximab and panitumumab. The pathophysiology may be related to the inactivation of EGFR expression in the ascending limb of the loop of Henle, where 70% of filtered magnesium is reabsorbed. Hypomagnesemia is a common adverse effect with up to 15% of patients manifesting severe magnesium wasting. The onset of hypomagnesemia can occur days to months after the initiation of therapy and may even present weeks after discontinuation of therapy. All patients should be followed closely for hypomagnesemia and appropriate magnesium repletion should be started as needed. Hypocalcemia is another electrolyte problem, which may need to be monitored while patients are on these agents.(30),(41)
Transient elevation in hepatic transaminases has been observed with the oral agents, gefitinib and erlotinib, and rare episodes of acute drug induced hepatitis have been reported. Dose reduction or treatment interruption are advised if the changes in liver function are severe.(39),(40),(47)
Conclusion
In summary, the underlying mechanisms that lead to dermatologic toxicities of EGFR inhibitors are poorly understood but are most likely from the inhibition of EGFR receptors in the skin. The follicular rash appears to be a good surrogate marker for response to therapy. Future controlled clinical trials are required to evaluate the various treatment modalities for evidence-based treatment of skin toxicity. The non-dermatologic side effects such as infusion reactions, interstitial lung disease, severe hypomagnesemia, etc., although less prevalent, demand early recognition because of their potential for critical outcome.