Course Authors
Jessica L. Mega, M.D., and David A. Morrow, M.D., M.P.H.
Dr. Morrow is Assistant Professor of Medicine, Harvard Medical School and Dr. Mega is a Cardiovascular Fellow, Harvard Medical School. Both are active investigators in the TIMI Study Group, Cardiovascular Division, Department of Medicine, Brigham & Women's Hospital, Harvard Medical School, Boston, MA.
Within the past 12 months, Dr. Morrow has received research grant support from Accumetrics, Amgen, AstraZeneca, Bayer Healthcare, Beckman Coulter, Biosite, Bristol-Myers Squibb, CV Therapeutics, Eli Lilly and Company, GlaxoSmithKline, Inotek Pharmaceuticals, Integrated Therapeutics, Merck, Merck-Schering Plough Joint Venture, Millennium Pharmaceuticals, Novartis Pharmaceuticals, Nuvelo, Ortho Clinical Diagnostics, Pfizer, Roche Diagnostics, Sanofi Aventis and Schering-Plough. He has been on the Speakers Bureau of Bayer Diagnostics, Beckman-Coulter, Dade-Behring, Sanofi Aventis and Roche Diagnostics, and been a consultant/advisor to Beckman-Coulter, Critical Diagnostics, Genentech, GlaxoSmithKline, Ortho Clinical Diagnostics and Sanofi Aventis. Within the past 12 months, Dr. Mega has received research grant support from Schering Plough.
This activity is made possible by an unrestricted educational grant from ![]() .
.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe pathophysiologic and clinical features of diagnoses across the spectrum of acute coronary syndromes
Discuss the role of risk stratification in the evaluation and management of patients with ACS
Evaluate the optimal approaches for reperfusion therapy for individual patients presenting with ST elevation MI
Integrate the evidence from clinical trials of antiplatelet and antithrombotic therapy into clinical practice.
Each year in the United States, 1.7 million patients are admitted to the hospital with acute coronary syndromes (ACS). The most common underlying mechanism is the rupture or erosion of atherosclerotic plaque inciting the formation of an obstructive thrombus.(1) During this process, the subendothelial matrix is exposed, leading to platelet adhesion and activation. Platelet aggregation occurs when fibrinogen molecules bind to the activated platelet receptors, cross-linking activated platelets. At the same time, plaque rupture leads to the release of tissue factor and activation of the coagulation cascade.(2) The resultant formation of an intracoronary thrombus can cause transient or persistent loss of blood flow to the myocardium, yielding ischemia or infarct, respectively (Figure 1).

Figure 1. Spectrum of Acute Coronary Syndromes.
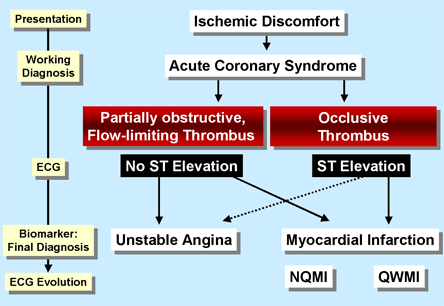
NQMI = non Q-wave myocardial infarction; QWMI = Q-wave myocardial infarction.

Clinically, patients presenting with characteristic chest pain that occurs at rest or with an unstable, accelerating pattern are evaluated for one of the three categories of ACS: ST elevation MI (STEMI), non-ST elevation MI (NSTEMI) and unstable angina (UA). Patients presenting with a STEMI have the highest likelihood of an acutely occlusive coronary thrombus within the culprit artery, with angiographic evidence demonstrating coronary thrombus in more than 90% of these patients.(3) In contrast, angiography performed in the acute period following UA/NSTEMI has shown the culprit artery to be free of occlusion in 60 to 85% of patients.(4),(5)
The distinction between UA and NSTEMI is made solely upon the presence of ischemia that is sufficiently severe and prolonged to result in sufficient myocardial damage to release detectable quantities of biomarkers of myocardial injury in patients with NSTEMI (Table 1).(6) From a perspective of their initial management, the two entities -- UA and NSTEMI -- are treated similarly and thus classified together as non ST-elevation ACS (NSTE-ACS).

Table 1. European Society of Cardiology/American College of Cardiology Biochemical Criteria for Myocardial Infarction.
To make the diagnosis of myocardioal infacrtion, the following must be associated with a clinical syndrome consistent with an ACS.
- Maximal concentration of troponin T or I exceeding the decision limit (99th percentile of the values for a reference control group) on at least one occasion during the first 24 hours after the index clinical event;
- Maximal value of CK-MB exceeding the 99th percentile of the values for a reference control group on two successive samples, or maximal value exceeding twice the upper limit of normal for the specific institution one occasion during the first hours after the index clinical event. Values for CK-MB should rise and fall; values that remain elevated without change are almost never due to an MI.

Risk Stratification in Acute Coronary Syndromes
The initial evaluation of the patient with a suspected ACS involves the concurrent assessment of the diagnosis (probability of ACS) and risk for complications (probability of death or recurrent ischemic events). The clinician must use a variety of data from the history, physical exam, electrocardiogram and other non-invasive data such as cardiac biomarkers to arrive at an overall assessment of the patient's risk (Figure 2).

Figure 2. Risk Stratification in ACS.
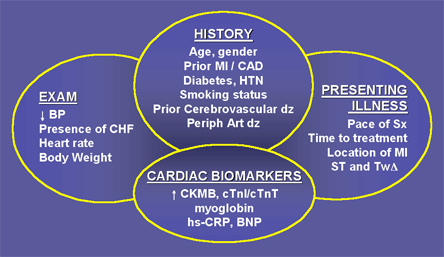
BP = blood pressure; BNP = b-type natriuretic peptide; CAD = coronary artery disease; CHF = congestive heart failure; CK-MB = creatine kinase-MB; DZ = disease; hs-CRP = high sensitivity c-reactive protein; HTN = hypertension; MI = myocardial infarction; SX = symptoms; TNI = troponin I; TNT = troponin t; TW = t-wave.

While their relative weighting in importance vary across the spectrum of ACS from unstable angina to STEMI, the key risk indicators are common across the entire syndrome, with advanced age and the presence of heart failure identifying patients at particularly high risk for fatal complications. Several simple tools for risk assessment have been developed and validated for risk prediction in patients with UA/NSTEMI (Figure 3) and STEMI (Figure 4).(7),(8),(9),(10)

Figure 3. TIMI Risk Score for UA/NSTEMI.
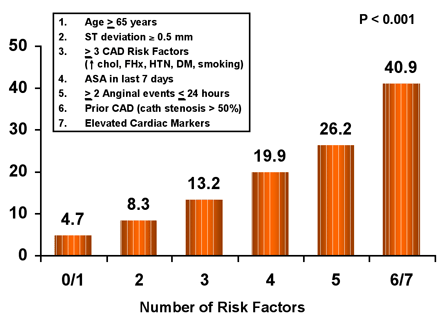
ASA = aspirin; CAD = coronary artery disease; CHOL = cholesterol; DM = diabetes mellitus; FHX = family history; HTN = hypertension.

Figure 4. TIMI Risk Score for STEMI: MOdel Calibration.
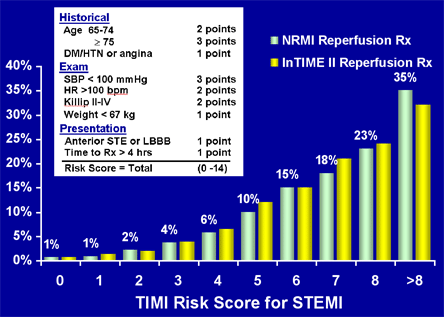
BPM = beats per minute; DM = diabetes mellitus; HR = heart rate; HRS = hours; HTN = hypertension; KG = kilogram; LBBB = left bundle branch block; NRMI = National Registry of Myocardial Infarction; RX = treatment; SBP = systolic blood pressure; STE = ST elevation.

Management
The management of ACS starts with early recognition of the syndrome and rapid evaluation, including acquisition of an ECG within 10 minutes, measurement of cardiac biomarkers, and administration of supplemental oxygen and aspirin.
STEMI
Fibrinolysis or Primary PCI
For patients with diagnostic ST-segment elevation, the fundamental goal of treatment is rapid initiation of reperfusion therapy with either primary percutaneous intervention (PCI) or fibrinolysis. The choice of lytic agents includes streptokinase, alteplase, reteplase and TNK-tPA.(11)(12)(13) With either reperfusion strategy, delay in treatment shows a strong relationship with subsequent short- and long-term mortality. For example, pooled data from over 1,700 patients indicate that for every 30-minute delay in symptoms to primary PCI, there is an 8% increase in 1-year mortality (Figure 5).(14) The ACC/AHA Guidelines for management of STEMI stipulate specific goals for initiation of fibrinolysis (30 minutes) or balloon inflation during primary PCI (90 minutes) relative to first medical contact.

Figure 5. Time-delay to Treatment with Primary Percutaneous Coronary Intervention and Mortality in Patients with STEMI.
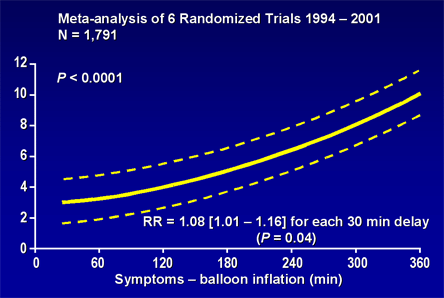

When primary PCI can be provided in a timely fashion by a skilled operator and experienced interventional team, this strategy provides superior outcomes to fibrinolysis.(15) Based upon these data, the ACC/AHA task force has given a Class I recommendation for primary PCI for patients with a STEMI who present within 12 hours of symptom onset and who can undergo the procedure within 90 minutes of presentation.(15) When a patient presents after 12 hours of symptom onset, primary PCI should be considered if the patient exhibits signs of heart failure, hemodynamic compromise or ischemic systems; however, a PCI-based reperfusion strategy may also be beneficial in stable, asymptomatic patients with delayed presentations.(16) The presence of cardiogenic shock, other high-risk features or contraindications to fibrinolysis also favors primary PCI as the preferred reperfusion strategy.
When primary PCI cannot be provided in a timely fashion by an experienced team, pharmacologic reperfusion therapy, including a fibrinolytic, should be administered. The efficacy of fibrinolysis is most pronounced when administered as early as possible, with the greatest benefit observed in patients presenting within the first two to three hours after symptom onset (Figure 6).(17) Thus, fibrinolysis may be the preferred reperfusion strategy among patients without contraindications who present within 3 hours of symptom onset and for whom the delay to providing primary PCI is expected to be longer than 60 minutes compared with administration of a fibrinolytic.(16) Absolute contraindications to administration include: prior intracranial hemorrhage, known structural cerebrovascular lesions, known malignant neoplasms, ischemic stroke within 3 months (except acute presentation), suspected aortic dissection, active bleeding, or significant closed-head or facial trauma within 3 months.(16) In patients presenting after 12 hours of symptom onset without ischemic symptoms, fibrinolysis is generally not utilized.

Figure 6. Saving Lives: Reducing Treatment Delay to Fibrinolysis.
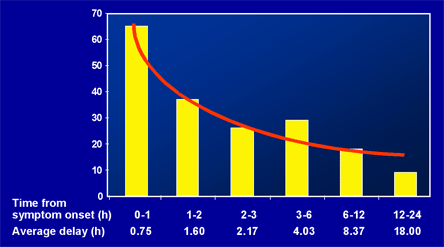
H = hours; No. = number; PTS = patients.

Antithrombin Therapy
In patients treated with either PCI or fibrinolysis for STEMI, antithrombin therapy is indicated as an adjunct to the primary reperfusion strategy. The 2004 ACC/AHA Guidelines for STEMI recommend (Class I) administration of intravenous unfractionated heparin (UFH) as a weight-based regimen (60 U/kg bolus up to 4000 U; 12 U/kg/hr maintenance up to 800 U/hr) for 48 hours in conjunction with fibrinolytic therapy. Since development of these guidelines, new evidence has become available regarding the use of alternative antithrombins in STEMI. Low-molecular-weight heparins (LMWH) such as enoxaparin and daltaparin have a mean molecular weight of 4,000 to 5,000 daltons and by virtue of a higher relative inhibition of activated factor X compared with UFH, interrupt the coagulation cascade at a higher point (Figure 7) with the potential to reduce the downstream amplification that occurs at each step in this enzymatic cascade.(18)

Figure 7. Coagulation Cascade.
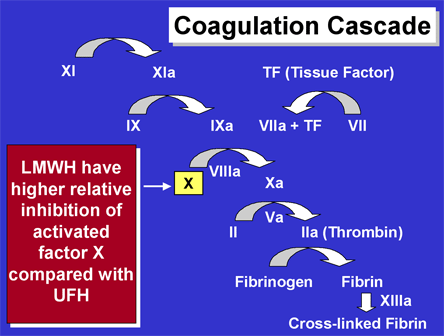

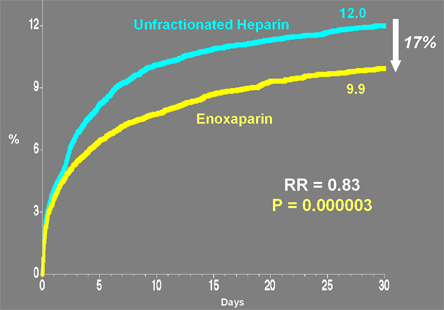
In addition, LMWHs have greater bioavailability, do not require monitoring of the aPTT and are less likely to induce immune-mediated thrombocytopenia.(19) Treatment with LMWH, as compared to UFH, as an adjunct to fibrinolysis does not appear to improve the rate of early reperfusion of the infarct-related artery but does reduce the risk of reocclusion and associated recurrent ischemic events (Table 2).(20),(21),(22),(23),(24),(25),(26),(27) In the randomized, double-blind, double-dummy, multinational ExTRACT-TIMI 25 Trial, enoxaparin administered for up to 8 days or until hospital discharge (whichever occurred first) reduced the rate of death or MI at 30 days by 17% compared to UFH given according to the 2004 ACC/AHA Guidelines (Figure 8).(28) A 0.7% absolute increase in major bleeding in the enoxaparin group was offset by the reduction in death or MI to result in a superior net clinical benefit with the enoxaparin strategy.

Figure 8. EXTRACT-TIMI 25: Primary Endpoint Death or Non-Fatal MI by 30 Days.

Antiplatelet Therapy
Because coronary plaque disruption leads to activation and aggregation of platelets, antiplatelet therapy plays a central role in the management of ACS including STEMI. In a collaborative meta-analysis that included 195 trials, antiplatelet therapy was shown to significantly reduce cardiovascular events, especially in high-risk patients.(29) In ISIS-2, the largest study of aspirin therapy in STEMI, an overall 23% reduction in mortality was observed in patients treated with aspirin.(30) Based on these data, current guidelines indicate that non-enteric coated aspirin should be administered to all patients with STEMI (in the absence of aspirin allergy) at doses between 162 to 325 mg and that chewing the medication promotes more rapid buccal absorption.(16)
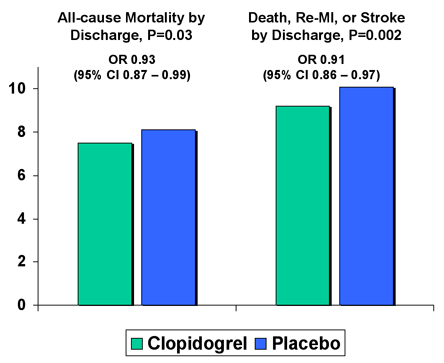
Important new data regarding the use of antiplatelet therapy have also become available since the formulation of the 2004 ACC/AHA Guidelines for Management of STEMI. Early treatment with clopidogrel in conjunction with aspirin and fibrinolytic in the randomized, placebo-controlled CLARITY-TIMI 28 Trial improved patency of the infarct-related artery and reduced ischemic complications (Figure 9).(31),(32) Likewise, in the COMMIT/CCS-2 Trial, treatment with clopidogrel following STEMI, as compared to placebo, reduced the rates of (1) death by hospital discharge and (2) death, non-fatal reinfarction or stroke by hospital discharge (Figure 10).(33) Of note, there was no difference in major bleed among the two groups (0.58% for clopidogrel and 0.55% for placebo, P=NS).

Figure 9. CLARITY-TIMI 28.
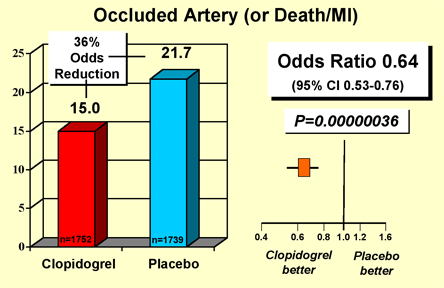

Figure 10. COMMIT - CCS2 Trial.

Inhibition of the platelet glycoprotein (GP) IIb/IIIa receptor with abciximab, tirofiban and eptifibatide has been evaluated in patients with STEMI. While combination pharmacological reperfusion with a GPIIb/IIIa inhibitor and a reduced dose of a fibrinolytic improves early patency, this combination is associated with an increased risk of bleeding and does not reduce mortality.(27),(34) In the setting of primary stenting for an acute MI, there is support for the use of IIb/IIIa inhibition.(35),(36)
Other Therapy
Beta-blockers should be administered early after presentation with STEMI, unless contraindications, including heart failure, are present. In a meta-analysis that included almost 29,000 MI patients, early treatment with beta-blockers resulted in a 13% relative reduction in the risk of mortality.(37) More recently, the COMMIT trial, which randomized 45,852 ACS patients (93% with STEMI or new LBBB) to metoprolol or placebo, suggested that the early beta-blocker therapy reduced rates of reinfarction and ventricular fibrillation but increased the risk of cardiogenic shock.(38) Thus, in hemodynamically unstable patients, it may be reasonable to defer upfront treatment with beta-blockade.
UA/NSTEMI
The majority of patients who present with symptoms that are concerning for an acute coronary syndrome but do not have ST-segment elevation on their ECGs are considered to have a non-ST elevation ACS. This group of patients includes those with definite myocardial injury (Figure 1 and Table 1) who receive the diagnosis of non-ST elevation myocardial infarction (NSTEMI), as well as those without myocardial injury who are defined as presenting with unstable angina (UA). Patients with UA and NSTEMI thus comprise a spectrum of patients with non-ST elevation ACS for whom the same strategies for evaluation and management are employed. This population of patients is very heterogeneous with respect to the risk of death and recurrent ischemic events. For this reason, a central principle of their management is to guide diagnostic evaluation and therapy according to the degree of risk.(7),(10)
The TIMI Risk Score for UA/NSTEMI provides an example of a tool that may be used to stratify risk and guide the selection of therapy. The TIMI Risk Score is calculated as the sum of the number of risk indicators that are present: (1) age >65; (2) three or more risk factors for coronary artery disease; (3) known significant coronary stenosis; (4) ST deviation >0.5mm; (5) elevated cardiac markers of necrosis; (6) severe anginal symptoms; and (7) use of aspirin in the prior seven days (Figure 3).(7) Patients are considered to be very high-risk if they have a TIMI risk score of 5 or greater and low-risk if the score is 2 or below. It has been observed that high-risk patients derive a greater benefit from early PCI and from the use of more potent anticoagulant and antiplatelet therapies (see below)(39)
Antiplatelet Therapy
Just as for STEMI, the foundation of treatment for UA/NSTEMI is the prevention of thrombus extension with antiplatelet and anticoagulant therapy. Trials evaluating the use of aspirin in UA/NSTEMI have consistently demonstrated a benefit when compared to placebo (Figure 11).(40)(41),(42),(43) The addition of clopidogrel to aspirin provides an additional 20% reduction in the risk of death, MI or stroke (Figure 12).(44) A benefit was conferred across the entire spectrum of risk in patients with UA/NSTEMI, and included patients managed medically and with coronary intervention.(45) The most recent guidelines from the AHA/ACC thus include a Class I recommendation to administer clopidogrel as soon as possible in patients undergoing medical management for non-ST elevation ACS and, at a minimum. at the time of angiography and, thereafter, in patients being managed with an early coronary angiography and percutaneous intervention. Optimally, clopidogrel should be discontinued five days prior to planned coronary artery bypass graft surgery.

Figure 11. Summary of Trials of Antithrombotic Therapy in UA/NSTEMI.
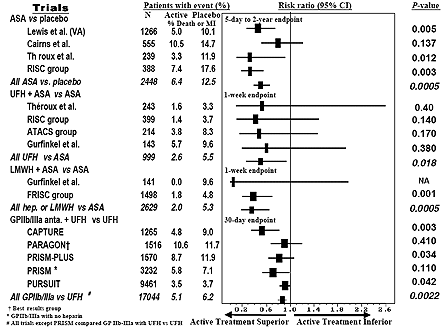
ASA = aspirin; UFH = unfractionated heparin.

Figure 12. CURE Trial.
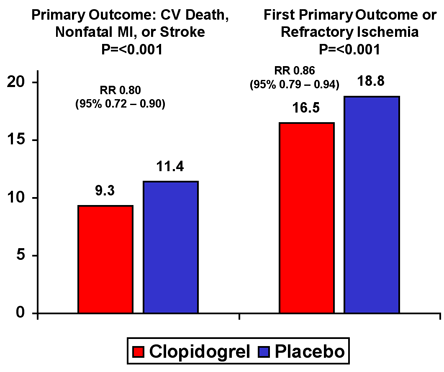

Glycoprotein (GP) IIb/IIIa receptor inhibitors, which prevent the final common pathway of platelet aggregation, also reduce the risk of death and recurrent ischemic events in patients with UA/STEMI. The benefit of GP IIb/IIIa receptor inhibition appears to be greatest with respect to the reduction of MI in patients undergoing percutaneous coronary intervention and has been demonstrated for abciximab, eptifibatide and tirofiban. Trials conducted in patients with UA/NSTEMI that included those being treated with and without early angiography and revascularization have shown improved outcomes with eptifibatide and tirofiban (Figure 13).(46),(47),(48),(49),(50) This benefit appears to be greatest in high-risk patients (Figure 14).(51) According to 2002 AHA/ACC Guidelines, GP IIb/IIIa inhibitors should be administered to patients undergoing PCI (Class I) or those who are at high-risk of recurrent events managed medically (Class IIa -- eptifibatide and tirofiban), including patients with elevated troponin levels, a TIMI Risk Score >4 or ongoing evidence of ischemia.(52),(53)

Figure 13. Randomized Trials of Platelet Glycoprotein IIb/IIIa Receptor Antagonists in Non-ST Elevation ACS.
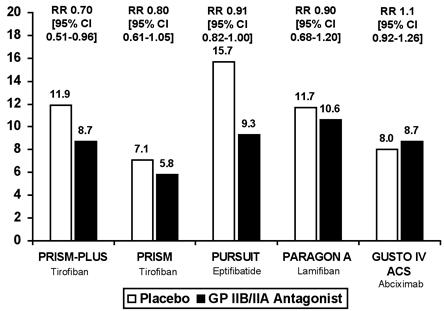

Figure 14. Effect of Tirofiban in Non-ST Elevation Acute Coronary Syndromes Stratified by Patient Risk and Therapeutic Strategy (PCI vs. No PCI).
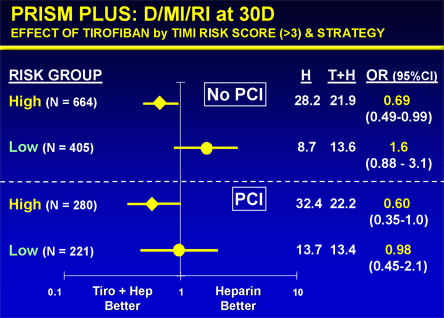
D = death; H = heparin; PCI = percutaneous coronary intervention; RI = recurrent ischemia; T = tirofiban.

Antithrombin Therapy
The AHA/ACC recommends administration of an antithrombin (unfractionated heparin or a low-molecular-weight heparin, Class I) for all patients with UA/NSTEMI. There are at least nine trials that have examined UFH and the risk of death or recurrent ischemic events in UA/NSTEMI patients. Although the findings have been somewhat inconsistent (Table 3), based on their overall trend and the pathobiologic understanding of the disease process, use of UFH has been a cornerstone of therapy for patients with UA/NSTEMI.(42),(52),(54),(55),(56),(57),(58),(59),(60),(61)
Low-molecular-weight heparins (LMWHs) have been compared to both placebo and to UFH for the management of patients with UA/NSTEMI. Overall, results from clinical studies support at least similar efficacy to UFH with more convenient subcutaneous dosing for LMWHs. At least three trials have shown enoxaparin to reduce the risk of death or recurrent ischemic events compared with UFH. For example, the TIMI 11B trial demonstrated a 24% reduction in the composite of death, non-fatal MI or urgent revascularization, evident as early as 48 hours compared to UFH. Trials that have included routine or more frequent early invasive management have indicated similar efficacy for enoxaparin and UFH (Table 4).(61),(63),(64),(65),(66),(67) On the basis of these findings, the 2002 ACC/AHA guidelines offer a Class IIa recommendation for the use of enoxaparin as a preferable alternative to UFH in UA/NSTEMI patients, unless CABG is planned or significant renal dysfunction is present.
Early Invasive vs. Conservative Management
Coronary angiography and revascularization, when appropriate, play an important role in the management of patients with UA/NSTEMI. There are two general strategies to the invasive evaluation and management of patients with UA/NSTEMI: early invasive and conservative. In the early invasive approach, patients without contraindication for catheterization are routinely referred for coronary angiography within 48 hours after presentation and revascularization when appropriate. The conservative strategy reserves cardiac catheterization for when ischemia recurs either spontaneously or with stress testing.
At least nine clinical trials have evaluated these two approaches. No significant difference between the early invasive and conservative strategies was observed in the first three trials;(68) however, subsequent trials, including FRISC II, TACTICS-TIMI18 and RITA 3, have shown a reduction in mortality and cardiovascular endpoints with the invasive approach (Figure 15).(39),(69)(70) The absolute and relative reduction in death or MI with the early invasive strategy is greatest in patients at high risk for recurrent ischemic events such as those with elevated troponin or a high TIMI Risk Score (Figures 16 and 17).(39),(71) Notably, in one trial, a routine invasive evaluation did not offer an advantage over a selective invasive management strategy in which more than 50% of the "selective" group underwent invasive evaluation during the initial hospitalization.(72),(73)

Figure 15. Routine Invasive vs. Selective Invasive Strategies in ACS.
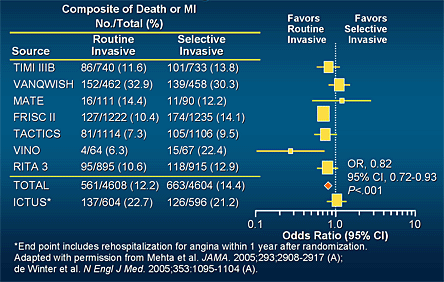

Figure 16. Rates of Death/MI in Troponin-negative and Troponin-positive UA/NSTEMI Patients Treated with Invasive and Conservative Management Strategies.
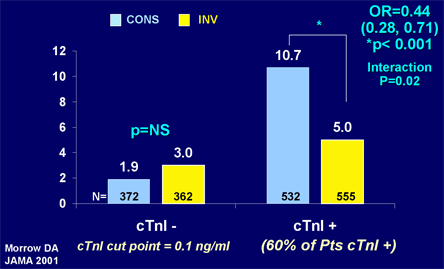
CONS = conservative; cTnI = Troponin I; INV = invasive; PTS = patients.

Figure 17.
Rates of Death/MI/ACS Rehospitalization Among UA/NSTEMI Patients with Low, Intermediate and High TIMI Risk Scores Treated with Invasive and Conservative Management Strategies.
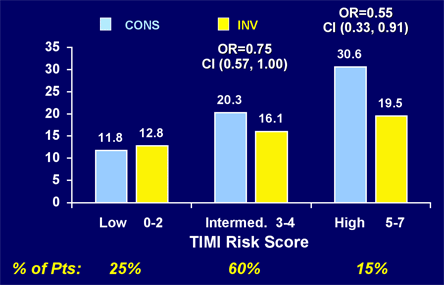
CONS = conservative; EP = endpoint; INV = invasive; MOS = months; REHOSP = rehospitalization.

Thus, ACC/AHA practice guidelines have given a Class I recommendation for early invasive strategy in patients with the following high-risk features: recurrent angina/ischemia at rest or with low-level activities despite anti-ischemic therapy; elevated TnT or TnI; new or presumably new ST-segment depression; recurrent angina/ischemia with CHF symptoms, an S3 gallop, pulmonary edema, worsening rales, or new or worsening mitral regurgitation; high-risk findings on non-invasive stress testing; depressed LV systolic function; hemodynamic instability; sustained ventricular tachycardia; PCI within 6 months; or prior CABG.(52) If these features are not present, then the guidelines support either an early invasive or conservative strategy.
Summary
The diagnosis of acute coronary syndrome (ACS) encompasses ST elevation MI (STEMI), non-ST elevation MI (NSTEMI) and unstable angina (UA). The initial evaluation of any patient suspected of having one of these three presentations includes simultaneous assessment of the diagnosis (probability of ACS) and risk for complications (probability of death or recurrent ischemic events).
For patients with diagnostic ST-segment elevation, the fundamental goal of treatment is rapid initiation of reperfusion therapy with either primary percutaneous intervention (PCI) or fibrinolysis. Likewise, coronary angiography and revascularization can play an important role in managing of patients with UA/NSTEMI; however, there are two general strategies of invasive management of patients with non-STE elevation ACS: early invasive and conservative.
The initial medical management of patients who present with both STEMI and UA/NSTEMI aims to prevent thrombus extension and consists of both antiplatelet agents (including aspirin, thienopyridines and GP IIb/IIIa inhibitors), as well as heparin or low-molecular-weight heparin products.
Finally, in all patients who present with an ACS, long-term risk factor modification, including intensive lipid management, is central to reducing early and late recurrent ischemic events.(74)