Course Authors
Mohammad Kamran, M.D., Bernard Hojaili, M.D., and Peter Barland, M.D.
Dr. Kamran and Dr. Hojaili are fellows in the Division of Rheumatology, Department of Medicine, the Albert Einstein College of Medicine and Montefiore Medical Center, New York.
Within the past 12 months, Dr. Kamran, Hojaili and Barland report no commercial conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe the possible mechanisms of action of IVIG as an immunomodulator
List rheumatological conditions where IVIG is of proven benefit
Discuss rheumatological conditions where IVIG is either only occasionally or questionably beneficial.
High doses of intravenous immunoglobulin (IVIG) purified from pooled normal human plasma were first used as an immunomodulating agent in1981 in idiopathic thrombocytopenic purpura (ITP).(1) Since then, IVIG has been employed in a wide variety of autoimmune and inflammatory diseases. In this Cyberounds®, we will review its use in several rheumatological conditions.
globulin G (IgG) mediates pro- and anti-inflammatory activities...
Mechanism of Action
Several possible mechanisms of action have been identified over the last two decades. These mechanisms may differ between diseases and even between subgroups of patients with similar clinical presentations. The postulated mechanisms of action include:
- IVIG contains anti-idiotypic antibodies that recognize antigenic epitopes in the variable antigen combining sites of other human immunoglobulins. These anti-idiotypic antibodies could bind to and neutralize pathogenic autoantibodies and prevent their interaction with the autoantigens.(2),(3) In addition, binding of the anti-idiotypic antibodies to surface IgM or IgG on B cells could generate negative signals on B cells resulting in down regulation of antibody production.(3)
- Immunoglobulin G (IgG) mediates pro- and anti-inflammatory activities through the engagement of its Fc portion of the molecule with distinct Fc receptors (FcRs) on the surface of several cell types including activated macrophages and endothelial cells. Engagement of Type I and Type III receptors results in a pro-inflammatory cytokine response, while engagement of Type II receptors is associated with an anti-inflammatory response. All IgG molecules contain a pair of identical oligosaccharides covalently linked to the constant region of their heavy chains within the Fc segment (Figure 1).

Figure 1. Immunoglobulin Structure.
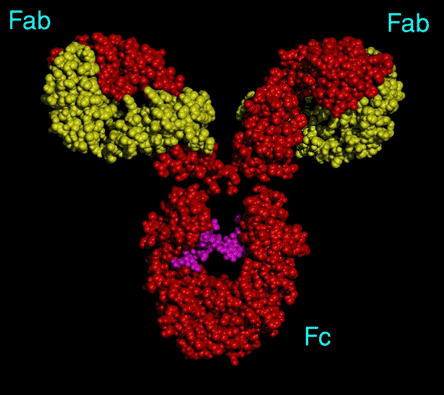
A typical IgG molecule. The red dots indicate the amino acids that make up the two identical heavy chains and the yellow dots depict the amino acids of the two identical light chains. The Fab portion of the molecule contains the antigen combining sites made up of variable regions of the heavy and light chains. The purple dots depict the polysaccharide side-chains that play a role in effector functions of IgG including the types of Fc receptors to which it can bind.

Recently, Kaneko et al. showed that those IgG molecules, which contain sialic acid in their oligosaccharides, react with and stimulate the production of Type II Fc receptors, leading to the production of anti-inflammatory cytokines. It is postulated that the monomeric IgG that predominates in IVIG is enriched in sialic acid and thereby promotes an anti-inflammatory and immunomodulating response.(4)
- c. IVIG is protective against complement-mediated tissue damage. IVIG interacts with activated complement components and prevents their deposition on target cells. In vitro studies have shown that IVIG inhibits the uptake of C3b and C4b complement fragments onto corpusculate immune complexes.(5)
None of these mechanisms of action is conclusive and different mechanisms may be dominant in diverse diseases and even between subgroups of the same disease spectrum.
Inflammatory Myopathies
Polymyositis (PM), dermatomyositis (DM) and sporadic inclusion body myositis (IBM) are three distinct idiopathic inflammatory myopathies that are characterized by progressive damage of striated muscle. Each is, however, distinguished by distinct clinical and histological differences.
Dermatomyositis
Dermatomyositis (DM) is characterized clinically by symmetrical proximal muscle weakness, photosensitive skin rashes and, in adults, by an increased incidence of malignancies. Although their efficacy has not been tested fully in randomized, placebo-controlled trials, corticosteroids, often in combination with an immunosuppressive agent such as methotrexate, azathioprine, cyclophosphamide or cyclosporine, are the standard initial treatments for DM.(6),(7),(8) However, 30-50% of patients are refractory to these agents.(9) In many of these refractory patients, children as well as adults, high-dose intravenous immunoglobulin (IVIG) has been shown to be beneficial.(10),(11) Dalakas et al.(12) treated 15 patients who were treatment resistant for at least for 4 to 6 months to high-dose steroids or therapeutic doses of another immunosuppressant (methotrexate, azathioprine or cyclophosphamide) in their double-blind, placebo-controlled crossover study. Eight patients who were initially assigned to the IVIG arm had significant improvement in scores of muscle strength (P<0.018) and neuromuscular symptoms (P<0.035). With crossovers, a total of 12 patients received IVIG. Nine of twelve patients treated with IVIG showed major improvement in their symptoms. There was no improvement or worsening in the placebo-treated patients. All patients continued to receive prednisone during the study. The IVIG effect became more significant after the second monthly infusion. Post-treatment muscle biopsies in the responders showed decreased intra-capillary complement deposition, diminished muscle necrosis, decreased endomysial lymphocytic infiltrates and improvement in the muscle cytoarchitecture.
We believe that IVIG given monthly is indicated in patients with active DM refractory to clinically safe doses of steroids and standard immunosuppressive therapy. Recent reports suggest that rituximab (see Extra Miles interactive program and previous Cyberounds®) may also be efficacious in this subset of patients.
Polymyositis
Clinically, polymyositis (PM) differs from DM by the absence of cutaneous manifestations and a diminished association with malignancy. Pathological differences include more myofibril degeneration and necrosis in PM. Like DM, corticosteroids and other immunosuppressive agents are first-line therapy with a significant number of patients refractory to either the initial or secondary courses of these agents.(9) Short-term, uncontrolled studies have shown that IVIG is efficacious in polymyositis. Cherin et al.(13) published an open prospective study of long-term follow up of IVIG therapy in 35 patients with chronic refractory polymyositis. Significant improvement in creatine kinase (CK), proximal muscle power, muscle disability scale score and esophageal disorders was observed in 25 patients. IVIG was discontinued after 3 months if no improvement was observed by then (10 patients). The mean follow up for the patients who responded favorably (25/35) was 51.4 + 13.1 months. Twelve patients remained in full remission (five with no drugs at all, seven with low-dose steroids) while seven improved but remained dependent on IVIG infusions. Seven patients relapsed at an average of 17.1 months after the discontinuation of IVIG. Patients with shorter duration of disease and higher baseline CK levels responded more favorably. Despite the absence of placebo-controlled, prospective long-term studies, it appears that IVIG therapy is a useful adjunct in the treatment of PM.
Inclusion Body Myositis
Inclusion body myositis (IBM) is characterized clinically as a slowly progressive myopathy occurring mainly in the elderly, presenting as proximal and distal weakness with modestly elevated levels of muscle CK. IBM is resistant to steroid and immunosuppressive therapy(14),(15) and the myocytes contain rimmed vacuolar inclusions.
There have been three controlled studies with use of IVIG in IBM. In the first controlled study by Dalakas et al.,(16) 19 patients with IBM were randomized to receive 3 months of IVIG or placebo in a crossover design. No statistically significant differences in the strength of the limb muscles were noted between the placebo and IVIG groups. However, significant improvement was observed regionally in some muscle groups in the IVIG randomized group. Walter et al.(17) randomized 22 patients to monthly infusions of IVIG or to placebo for 6 months each, followed by the alternative treatment. Overall, there was no progression of the disease in 90% of the patients and no differences between groups at 6 months. A mild, yet statistically significant, improvement (P<0.05) in clinical symptoms was found using the Neuromuscular Symptom Score (NSS) but not with other functional evaluations. In a third trial,(18) 36 patients with biopsy-proven IBM were randomized to receive IVIG or placebo monthly on 60 mg/d prednisone background for 3 months. No significant changes in muscle strength were noted between the two groups. Thus, IVIG does not appear to be beneficial in IBM.
Kawasaki's Disease
Kawasaki's disease is a febrile illness of childhood. It is a self-limited, acute, vasculitic syndrome of unknown etiology characterized by cervical adenopathy and tongue inflammation. Approximately 25% of untreated children develop cardiovascular sequelae ranging from asymptomatic coronary artery ectasis or aneurysm formation to giant coronary artery aneurysms with thrombosis, MI and sudden death. Combined therapy with aspirin and high-dose IVIG (2 g/kg), initiated in the first 10 days of illness, reduces the occurrence of coronary artery aneurysm from 20-40% to 4% at 60 days after the onset of the illness.(19),(20) There have been more than 21 randomized trials(22) and 3 meta analyses(19),(20),(21) which have clearly established the efficacy of IVIG in reducing the cardiac sequelae of Kawasaki's disease. A single infusion of 2 g/kg IVIG was more effective than divided doses of 400 mg/kg/day for 5 consecutive days in the prevention of complication at 30 days.(19)
Systemic Lupus Erythematosus
In systemic lupus erythematosus (SLE), the value of IVIG is not well established. Most of the data are from case reports and small series involving SLE predominantly in a single organ system.(22) Renal disease in systemic lupus erythematosus is a cause of significant morbidity and mortality. Conventional treatment for proliferative lupus glomerulonephritis remains corticosteroids plus mycophenolate or cyclophosphamide. Uncontrolled studies involving a small number of patients have shown that IVIG is occasionally effective in membranous and proliferative lupus nephritis that is resistant to conventional regimens.(23),(24) Repeated courses may be required(25) and cost may be an issue.
SLE is frequently associated with autoimmune-mediated thrombocytopenia. There is no established standard treatment for thrombocytopenia in SLE, as the literature consists of small case series or isolated case reports. Corticosteroids are considered first-line treatment, while the long-term efficacy and safety of splenectomy remains controversial. Though many patients respond initially to steroids, relapses are frequent when the dose is tapered.(26)
Intravenous immunoglobulin rapidly raises the platelet count in SLE-associated thrombocytopenia. However, most studies recommend limiting IVIG to patients with life-threatening thrombocytopenia refractory to steroids. Arnal et al.(26) evaluated IVIG treatment in severe immune thrombocytopenia associated with SLE. Intravenous immunoglobulin was given to 31 patients in a dose of 2 g/kg over 2 to 5 days. A transient response was demonstrated in 20 patients (65%) but no sustained response could be observed, even when repeated IVIG infusions were provided to four patients. In another study, 7 SLE patients were treated with IVIG, 5 had a more than 50% increase in their platelet counts and 4 of them had a sustained benefit for at least 6 months.(27)
There are only anecdotal reports of IVIG therapy for CNS manifestations of SLE. Winder et al.(25) 25 reported a successful outcome in a patient with lupus pneumonitis and encephalitis unresponsive to corticosteroids and conventional immunosuppressive therapy. The patient received continuous treatment with IVIG, every 4 weeks for up to 20 months, which induced a prolonged clinical and laboratory remission. There have been other reports of successful treatment but no long-term follow-up. Since case reports are usually biased towards successful outcomes, it is very difficult to assess the utility of IVIG in most manifestations of SLE, though it appears to have a short-term role in severe thrombocytopenia.
Vasculitis
Wegener's granulomatosis, eosinophilic granulomatosis and angiitis (Churg-Strauss syndrome) and microsopic polyangiitis are systemic necrotizing vasculitides that usually involve the small and medium-sized arteries of the respiratory system and kidneys and are characterized by the presence of circulating anti-neutrophil cytoplasmic antibodies (ANCA). Most patients with ANCA-associated vasculitis can be successfully treated with a combination of oral steroids and cyclophosphamide. However, a significant number of patients are refractory to these agents.
In a small, prospective, double-blind study, 34 patients with chronic active ANCA-associated vasculitis who had received at least two months of conventional therapy with continued disease activity were randomized to receive either one course of IVIG (400 mg/kg/d for 5 days) or placebo while still receiving standard therapy. After one month, improvement was noted in 14 out of 17 patients in the IVIG arm, compared with no improvement in the patients receiving placebo. The efficacy of the single course of IVIG was transient, since there was no difference between the groups after 3 months.(28)
Data about the efficacy of IVIG in Churg-Strauss syndrome mostly come from case reports.(29),(30) The first open label prospective study of a total of 18 patients with Churg-Strauss syndrome investigated the effect of synchronized cycles with plasmapheresis and IVIG repeated monthly for 6 months and every other month for 3 cycles in 9 patients in comparison with the standard therapy (1 mg/kg/d of prednisone and 2 mg/kg/d of cyclophosphamide). Significant differences in the Birmingham vasculitis activity score, the daily maintenance prednisone dose and the remission rate were achieved at the end of 3 years in the treatment group.(31) Tsurikisawa et al.(32) reported significant improvement in neuropathy and heart failure within a few weeks of IVIG in an open label study of 15 patients with Churg-Strauss syndrome who initially responded to steroids with or without other immunosuppressive therapies but had heart failure, secondary to vasculitis, and persistent neuropathy.
Systemic Sclerosis
Systemic Sclerosis (SSc) is a chronic disorder of unknown etiology characterized by excessive deposition of collagen and other extracellular components in the skin and in visceral organs and an occlusive vasculopathy. While IVIG has been reported to improve the Rodnan skin score in one small open label study,(33) the general impression among rheumatologists experienced in treating SSc is that IVIG is ineffective.
Anti-Phospholipid Syndrome in Pregnancy
Anti-phospholipid antibodies (APA) have been shown to be risk factors for intrauterine death, growth retardation, premature birth, pre-eclampsia and repeated fetal loss in patients with SLE,(34),(35) as well as in women with no underlying autoimmune disease.(36) The standard therapy for pregnant women with a history of previous obstetrical complications associated with APA is low-molecular-weight heparin and low-dose asprin.(37)
The use of IVIG in APA pregnancy is not well established. A randomized study of 40 patients by Triolo G et al.(37) found IVIG to be less effective than heparin plus low-dose aspirin. Women treated with LMW heparin plus low-dose aspirin had a higher rate of live births (84%) than those treated with IVIG (57%). The same group reported, in another retrospective analysis, that IVIG treatment was followed by successful pregnancy outcome in 8 of 10 women who in their prior pregnancies had failed therapy with heparin plus low-dose aspirin.(38)
Sjögren's syndrome
Sjögren's syndrome is an autoimmune inflammation of the salivary and lacrimal glands. As discussed previously on Cyberounds®, the syndrome may present with several extra-glandular features including vasculitis, as well as central and peripheral neurological lesions. There is a paucity of published data concerning the efficacy of IVIG in Sjögren's syndrome. Takahashi et al.(39) reported improvement in 4 out of 5 cases of ataxic sensory neuropathy with Sjögren's syndrome treated with IVIG. It has also been used with favorable results in Sjögren's associated vasculitis.(40)
Henoch-Schönlein Purpura
Henoch-Schönlein purpura (HSP) is one of the most common systemic vasculitis in children and has a prominent cutaneous component. This disease predominantly involves the post-capillary venules, capillaries and arterioles and is associated with deposition of IgA-containing complexes in the pathological lesions. Clinical manifestations include a classic tetrad of rash, arthralgias, abdominal pain and renal disease that can occur in any order and at any time over a period of several days to several weeks. The use of high- or low-dose intravenous immunoglobulin has been shown in limited studies to stabilize poor renal function or slow progression of HSP nephritis.(41),(42)
Conclusion
Currently, the use of IVIG is approved for primary immune deficiencies, CLL with hypogammaglobulinemia and/or recurrent bacterial infections, hematopoietic stem-cell transplantation (allogenic) age >20 years, Kawasaki's disease, idiopathic thrombocytopenic purpura and pediatric HIV type 1 infection. The off label use of IVIG, according to some reports, exceeds its approved use.(43)
IVIG is the drug of choice in Kawasaki's disease, and is beneficial in dermatomyositis and polymyositis with good supporting evidence. Its efficacy has not been well established in anti-phospholipid syndrome in pregnancy and Sjögren syndrome. It should be considered as a second-line or third-line treatment in SLE, Henoch-Schönlein purpura and vasculitis. It has not been shown to be effective in inclusion body myositis. IVIG is very expensive and its appropriate use is important but difficult to resolve at this time.