Course Authors
Sheryl Merkin, M.S., F.N.P., C.D.E., Sharon Movsas, M.S., R.D., C.D.E. and Joel Zonszein, M.D., C.D.E.
Release Date: 07/24/2006
Upon completion of this Cyberounds®, you should be able to:
State the recommendations for glucose monitoring and diet in individuals receiving intensive insulin therapy
Explain the basic principles of insulin replacement hormone utilizing basal/bolus regimens
Identify the new insulins and new insulin delivery systems.
Case Report As we know from the previous Cyberounds Endocrinology, this is a 51-year-old, white, male with new-onset diabetes. He had a strong family history of diabetes, his BMI was 17 kg/m2, he was normotensive and had hyperchlestrolemia treated with atorvastatin 10 mg daily; his triglycerides and HDL-cholesterol were normal. The patient did not have evidence of any chronic diabetic complications. Laboratory results showed an initial HbA1c of 11%, a low C-peptide (0.7 ng/ml) and high antibodies to glutamic acid dehydrogenase (anti-GAD) (49.5 U/ml). The diagnosis of Latent Autoimmune Diabetes of the Adult (LADA) was established. The patient was treated initially with a regimen consisting of fixed doses of Novolog mix 70/30® pen syringe (recombinant DNA human insulin with 70% protamin aspart suspension and 30% soluble aspart insulin) before breakfast and before dinner. With this regimen, he developed several hypoglycemic episodes. Because of inadequate counseling and education by the initial physician, the patient decided to change endocrinologists. He was seen by a second endocrinologist who believed in the team approach and who worked with a nurse and nutritionist who were both Certified Diabetes Educators (CDE). After evaluation and education, the insulin regimen was changed to Lantus® glargine given in the morning and the patient was instructed to self-adjust the dose according to his pre-meal blood sugars. On this regimen, his blood glucose values improved significantly. He felt well, resumed his usual work hours and sports activities without further episodes of hypoglycemia. He came for a follow-up visit to review his insulin regimen. |
A. Metformin, an oral agent that decreases hepatic glucose production, has no indication in the treatment of Type 1 diabetes.
B. Nateglinide, an oral agent that causes insulin secretion, has no indication in the treatment of Type 1 diabetes.
C. Pioglitazone, an oral agent that causes insulin sensitivity, has no clear role and or indication in the management of individuals with Type 1 diabetes.
D. Glimepiride, an oral agent that causes insulin secretion, has no indication in the treatment of Type 1 diabetes.
E. None of the above is correct. The patient has improved insulin secretion and better glycemic control as a result of insulin therapy and resolution of glucose toxicity. This window of improvement in glycemic control is also known as the "honeymoon period of the disease," tends to vary in time, but is often associated with mild hyperglycemia that can cause chronic complications (1),(2),(3),(4),(9) and less islet β-cell function later in the course of the disease.(6) Delaying intensive insulin treatment, therefore, will be conterproductive in these patients. This strategy needs to be explained as part of the necessary education for individuals with Type 1 diabetes, i.e., intensive insulin therapy is recommended.
After the Diabetes Control and Complications Trial (DCCT) findings showed that improved glycemic control with intensive insulin therapy in patients with Type 1 diabetes mellitus led to significant reductions in retinopathy, nephropathy, neuropathy(2), and cardiovascular disease,(1) achieving glycemic control as close to normal (A1C 6-7%) without meaningful hypoglycemia has become the standard of care.
Insulin is available in rapid-, short-, intermediate- and long-acting formulations that can be injected separately or mixed in the same syringe. Rapid-acting insulins are available as analogues (insulin lispro, insulin aspart, insulin glulisine), and recently a non-injectable rapid acting inhaled insulin, (Exubera® was approved for clinical use. These insulins better mimic naturally-secreted meal insulin bursts when compared to Regular insulin; long-acting insulins are also available as analogues (glargine and detamir), and are slowly replacing the intermediate-acting insulins such as lente and NPH, and the long-acting insulin, ultralente. The long acting insulin analogues are more predictable with less variability in glycemic changes.
Insulin preparations with a predetermined proportion of intermediate-acting insulin mixed with short- or rapid-acting insulin (e.g., 70 percent NPH/30 percent regular, 50 percent NPH/50 percent regular and 75 percent NPL (protaminized insulin lispro)/25 percent insulin lispro) are also available and are shown in Table 1.

Table 1. Fixed-Mixed Insulins.
Humulin (NPH/regular) 70/30 and 50/50 (NPH/regular) Humalog 75/25 (Prot-lispro/free lispro) Novolin 70/30 (NPH/regular) Novomix 70/30 (Prot-aspart/free aspart) |
Table 1 lists human insulins. Shown in green are "designed insulin analogues." The percentage of rapid or very rapid insulin is shown as the denominator.

Table 1a. New and Commonly Used Insulins: Basal and Bolus.
| Peak | (duration) hrs | ||
|---|---|---|---|
| Rapid Acting | |||
| Humalog lispro | 1-2 | (2-6) | |
| Novolog aspart | 1-2 | (2-6) | |
| Apidra glulisine | 1-2 | (2-6) | |
| Short-Acting | |||
| Regular | 2-4 | (3-6) | |
| Intermediate-Acting | |||
| NPH | 6-12 | (10-24) | |
| Long-Acting | |||
| Lantus glargine | none | (24) | |
| Levemir detemir | none | (12-24) dose related |
|
All human insulins, shown in green, are "designed insulin analogues" produced by a process that uses recombinant DNA to produce an insulin molecule that is different from human insulin in structure as well as pharmacokinetic/pharmacodynamic properties.

Conventional insulin therapy regimens using a single daily injection, or two injections per day (regular and NPH insulin, mixed together in the same syringe, given in fixed amounts before breakfast and dinner) is now replaced for insulin regimens providing a basal bolus approach. The stable basal insulin is given as one to two daily injections of intermediate- or long-acting insulin, or via continuous subcutaneous insulin infusion (insulin pump). Bolus insulin -- regular insulin, rapid-acting insulin analogues or the recently FDA-approved rapid-action inhaled insulin (Exubera®) -- is given in addition to the basal insulin before each meal. The choice of regimen and type of insulin is individualized and depends on each patient's lifestyle and preference.(11) For optimal insulin regimens, understanding insulin pharmacokinetics is critical, as the duration of action is affected by such factors as the type of insulin preparation, insulin dose, injection technique and site of injection.(12)
Patient education about insulin addresses a few basic issues -- unused vials should be refrigerated and both extreme temperatures and excess agitation avoided -- to preserve its potency. When clumping, frosting or precipitation are noticed, the insulin should be replaced. An extra bottle of each type of insulin used should always be available.
Conventional insulin administration involves injection with syringes that are now available as 0.3 cc (up to 30 units), 0.5 cc (up to 50 units), and 1 cc (up to 100 units) with smaller (30 and 31 gauge) needles of several lengths. Prescriptions should specify the type of syringe and needle. When pen syringes are used instead of the classical syringes, prescriptions for the pen syringe, as well as the pen needles, also need to be specific. Insulin syringes and pens, needles and lancets should be disposed properly either through needle disposal programs or placed in a puncture-resistant container; local trash authorities should be contacted for proper disposal and to assure that these containers will not be recycled.
Basal Insulins: Intermediate and Long-Acting
Exogenous basal insulin is provided continuously throughout the day with the goal of normalizing non-meal related blood sugars. Although it is meant to resemble the sustained non-meal related endogenous insulin, a true physiologic replication is extremely difficult. Also, insulin is administered peripherally and not via the portal system that is the case with endogenous insulin that is constantly changing in response to the provision of fuels and the regulation of many other hormones. Thus exogenous insulin with fixed absorption rates and duration of action cannot replicate the intricate endogenous insulin secretion.
Until recently, NPH has been the classic basal insulin, used widely in DCCT.(2) In Type 1 diabetes, NPH insulin should be given as two injections -- in the morning and at bedtime. The bedtime dose attempts to take advantage of its peak -- the maximum insulin oncentration/effect that occurs 6-7 hours later -- to meet the increased insulin demands that take place in the morning (dawn phenomenon). When given before dinner NPH may cause hypoglycemia as it typically peaks around 3 AM, the same time of the physiologic glucose nadir. In practice, however, NPH peaks are difficult to predict because of high intrapatient and interpatient variability. Thus, the two new analogues (glargine and detemir) provide more steady basal replacement, resulting in less hypoglycemia and less hyperglycemia. Though they offer better control, they still do not replicate physiologic secretion of basal endogenous insulin production.
Lantus® (glargine) insulin was the first insulin analogue with a prolonged duration of action to become available. Insulin glargine differs from human insulin in that the amino acid asparagine at position A21 is replaced by glycine and two arginines are added to the C-terminus of the β-chain. These amino acid changes reduce the aqueous solubility of insulin glargine at physiologic pH, stabilizing the hexamers and delaying its dissociation into monomers. It is released gradually from the injection site with a slow absorption which results in a relatively constant basal insulin supply. Glargine is formulated as a clear acidic solution and because of its low pH cannot be mixed with other insulins.(13) Therefore, a basal/bolus regimen using glargine requires 4 injections a day compared to a basal/bolus regimen using NPH which requires 3 injections a day.
While NPH insulin has a peak action at 6-7 hours after injection, with detectable serum insulin concentration levels after 17 hours, insulin glargine only has a small peak at four hours and remains active at 24 hours. Studies comparing NPH twice-daily plus two pre-meal boluses at breakfast and dinner to glargine once-daily plus three pre-meal boluses have shown that the glargine regimen has fewer hypoglycemic episodes, particularly nocturnal hypoglycemia, lower fasting blood glucose concentrations and produces the same or slightly improved A1C values.(14),(15) While many patients achieve stable basal serum insulin concentrations with a single daily injection of glargine, given in the morning or evening (it is approved to be given at any time of the day), some patients with Type 1 diabetes may need twice-daily insulin injections.
Levemir® (insulin detemir, rDNA origin), approved by the FDA in June 2005, is the second long-acting insulin analogue available.(16) The prolonged action of this insulin is attributable to the addition of a fatty acid side chain that allows a constant albumin binding and unbinding which results in a steady insulin serum concentration and less variability of blood glucose concentration. The duration of action is dose-dependent but appears to be shorter than that of insulin glargine. Levemir® has been approved by the FDA to be used once or twice daily in patients with Type 2 or Type 1 diabetes. Glycemic control appears to be similar in trials comparing insulin detemir and NPH. However, insulin detemir is associated with less nocturnal hypoglycemia, better fasting blood sugars and less weight gain.(17),(18),(19)
E is correct. Although the patient's glycemic control improved significantly and he was doing fine with basal insulin only, evidence based medicine supports a more aggressive approach in order to reduce the risk of diabetic retinopathy, nephropathy, neuropathy(2),(3),(6),(7),(8) and macrovascular complications.(1) Intensive therapy markedly reduces the risk of progressive retinopathy and is more effective when introduced during the first five years.(2) The mechanisms by which early intensive insulin management prevents long-term complications known as the "metabolic memory" are unclear;(9) any elevation in glycemia, even in the subdiabetic range, can increase the risk for cardiovascular disease.(10) Thus, implementation of intensive insulin therapy as early as possible and maintenance of good glycemic control as long as possible result in a short- and long-term reduction of complications.(1),(2),(3),(6),(7),(8),(9),(10) Also early intensive insulin treatment results in better metabolic control short and long term, probably by preserving beta cell function.(12)
A is incorrect. Changing from glargine to detemir at bedtime will not improve glycemic control. Detemir works for 12 hours, thus two injections are often required. The advantages of switching to detemir are less hypoglycemia, less weight gain and improvement in fasting blood glucose, none of which was an issue at this time in this case.
B is incorrect. Changing from glargine to two injections of detemir will not improve glycemic control. While advantages of switching to Levemir are less hypoglycemia, less weight gain and improvement in fasting blood glucose, none of which was an issue at this time in this case.
C is incorrect. Glargine, a basal insulin, cannot prevent postprandial glycemic surges in insulinopenic individuals. Sooner or later, the patient will need to add pre-meal bolus injections. There is plenty of evidence that earlier and more aggressive treatment will result in fewer complications and better glycemic control.(1),(2),(3),(6),(7),(8),(9),(10)
D is incorrect. This patient already has an effective basal regimen. Switching to two injections of NPH will cause more glucose variability, because of the NPH peaks, so that a bolus regimen will still be necessary.
Case Report, Continued During a follow-up visit with the nurse, blood glucose records showed significant improvement but, based on his diagnosis of Type 1 diabetes, a more intensive regimen was indicated. After education was provided on the intensified regimen, including relevant changes in glucose testing, insulin administration and record-keeping, a basal-bolus regimen was prescribed. The patient was resistant to intensifying the regimen as it meant more injections requested information about Exubera®, the inhaled insulin recently approved by the FDA. However, since it was not yet available in the market, he agreed to start a basal bolus regimen with a fixed dose of rapid-acting Apidra®, 3 units before each meal. The basal insulin was reduced from 12 to 8 units daily to avoid hypoglycemia because, typically, basal insulin is about 40-60% of the total daily dose. He was asked to fax weekly blood glucose records and was referred to the dietitian for carbohydrate counting, a skill needed for self-adjustment of pre-meal insulin boluses according to the meal. |
A more intensive insulin regimen is necessary not only to avoid chronic diabetic complications but also for preservation of beta cell function and easier management.(12) Since the patient has adequate basal insulin, bolus insulin will improve postprandial glycemias. Bolus insulin doses change constantly and need to be calculated by the patient according to the blood sugar level (correction factor), carbohydrate to insulin ratio (CIR) related to insulin sensitivity and carbohydrate content of each meal. Since education for self insulin adjustments takes some time, he was initially prescribed a fixed dose.
Bolus Insulins: Rapid-acting Analogs, Regular and Inhaled
In addition to regular insulins, there are now three rapid-acting injectable insulins and one inhaled insulin that can be used for pre-meal boluses. Rapid-acting insulin is designed to have a faster onset and shorter duration than regular insulin. Modifications made to the insulin molecule, by substituting an amino acid(s) in the β-insulin chain, permit faster absorption with an onset of action in 5 to 15 minutes, peak action at 30 to 90 minutes and duration of action of 2 to 4 hours (Table 2).
Table 2. New and Commonly Used Insulins: Basal and Bolus
| Peak | (duration) hrs | |
|---|---|---|
| Bolus | ||
| Rapid Acting | ||
| Humalog lispro | 1-2 | (2-6) |
| Novolog aspart | 1-2 | (2-6) |
| Apidra glulisine | 1-2 | (2-6) |
| Short-Acting | ||
| Regular | 2-4 | (3-6) |
| Basal | ||
| Intermediate-Acting | ||
| NPH | 6-12 | (10-24) |
| Long-Acting | ||
| Lantus glargine | none | (24) |
| Levemir detemir | none | (12-24) dose related |
All human insulins, shown in green, are "designed insulin analogues" produced by a process that uses recombinant DNA. The recombinant DNA is used to formulate an insulin molecule that is different from human insulin in structure as well as pharmacokinetic/pharmacodynamic properties.
Compared to regular insulin, rapid-acting insulins allow easier timing with meals, achieve better reductions in postprandial hyperglycemia and provoke fewer instances of hypoglycemia.(20) But they also may result in hypoinsulinemia and hyperglycemia 5 to 7 hours after injection due to their shorter duration of action.(21) When this occurs, it is necessary to increase the dose of the intermediate or long-acting insulin.
Rapid-acting insulins are more convenient for patients using intensive insulin therapy, as they correct hyperglycemia more rapidly than regular insulin. These effects appear to be equally effective in the three analogues.(22),(23),(24) Results from clinical trials show only small differences in the reduction of A1C between the analogues and regular insulin.(25) Rapid-acting insulins cost more, and the possibility of teratogenicity and long-term safety are unknown.
Humalog® {lispro insulin [Lys(B28), Pro(B29)]-human insulin} was the first commercially available analogue. Lispro is identical to human insulin except for a reversal of the 28th and 29th amino acid residues (proline and lispro, respectively) of the normal insulin _-chain, permitting it to act similarly to monomeric human insulin. Lispro has the pharmacokinetic profile of the rapid acting insulins and thus better mimics the normal prandial insulin surge in response to carbohydrate ingestion.(26)
Novolog® [aspart insulin (B28 Asp-human insulin)] was the second rapid-acting analogue, engineered through replacement of the proline residue at position 28 of the β-chain with aspartic acid. The replacement decreases self-association of insulin aspart monomers, resulting in a pharmacokinetic profile similar to lispro's, and it is, thus, well suited for use as meal insulin.
Apidra® (glulisine insulin, rDNA origin) was FDA approved in April 2004 and recently marketed.(27) This rapid-acting insulin analog differs from human insulin by the replacement of the amino acid asparagine with lysine at position 3 and lysine with glutamic acid at position 29 of the β-chain. Compared with regular insulin, and similar to other rapid analogues, glulisine has a more rapid onset of action and a shorter duration of action. Glulisine offers greater treatment convenience and requires less mealtime planning (compared with regular insulin). It has been approved, therefore, for administration immediately before the meal or with the meal.(27),(28),(28)
Exubera® [Insulin Human (rDNA origin)], is the first non-injectable insulin that can be administered in an inhaled version. It was approved by the FDA in January 2006 for treatment of adult patients with Type 1 and Type 2 diabetes and it will be marketed by July 2006. Until now, injections have been the only way to deliver insulin and while advances in syringe and needle technology, insulin pens and pumps have enhanced insulin delivery, many doctors and patients welcome alternatives to injection.(30)

Figure 1a. Advantages of Insulin Pens.
- Convenience and accuracy for the patient and the physician
- Less patient anxiety
- Less need for dexterity
- Sharper needles with less discomfort
- Increased patient satisfaction
- increased compliance?

Figure 1b. Illustration of Two Insulin Pumps.

Continuous Subcutaneous Insulin Infusion (CSII)
Size - 3.0" x 2.0" x .76" (Smaller than a business card)
Weight - 3.03 oz (with battery)

Figure 1c. Illustration of a New Insulin Inhaled Device
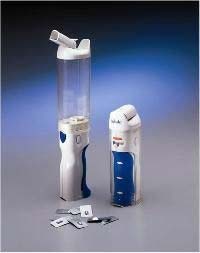
This smaller insulin inhaler device has not yet been approved for use in this country.

Exubera® is a rapid-acting, fine dry-powdered insulin that enters the bloodstream more rapidly than by subcutaneous injection and, thus, may prove especially beneficial in pre-meal insulin administration; it does not replace longer-acting insulins. Exubra® is dispensed in 1 mg (equivalent to 3 units of regular insulin) and 3 mg (equivalent to 8 units of regular insulin) blister packs. Individuals with Type 1 diabetes treated with Exubera® combined with a single nighttime injection of long-acting insulin provided glycemic control comparable to that achieved with a conventional subcutaneous insulin regimen.(31) Inhaled insulin was associated with improved patient satisfaction and quality of life, which may help patients accept intensive insulin regimens.
Although inhaled insulin may be a valuable noninvasive component of therapy for T1DM, only a small percentage of the inhaled insulin actually reaches the bloodstream. Large amounts are, therefore, needed and much is "wasted," resulting in the relatively high cost. While the large surface area of the lung enhances absorption of insulin, safety remains a concern. In general, frequency and nature of adverse events were similar in the Exubera® and control groups. The most common adverse events were hypoglycemia, cough and bitter taste. As Exubera® may affect lung function, patients need to have their lungs tested before starting the medication, and then every 6 to 12 months thereafter as directed by a healthcare provider. Exubera® is not recommended in people who smoke, recently quit smoking or who have chronic lung disease (such as asthma, chronic obstructive pulmonary disease or emphysema). In addition to inhaled insulin, there are other non-injectable delivery systems being investigated such as transdermal, buccal and oral insulins.
Fixed combinations insulins are commercially prepared mixtures where the basal and bolus insulins are divided with the intention (unsuccessful) of replicating physiological insulin production. While they serve a purpose, especially initially in patients who require a simpler and more convenient method of physiologic insulin replacement, glycemic control is often hard to achieve because they limit optimal individualized basal-bolus needs.(5) Novolog 70/30, for example, is a fixed combination (70% of protamin aspart suspension and 30% soluble aspart insulin; all recombinant DNA human insulin) that is given twice daily before breakfast and dinner at a prescribed dose without self-adjustment. While simpler to implement, this type of regimen is associated with long-term weight gain and hypoglycemia, both of which often lead to compromised adherence. As the name suggests, "fixed combinations" limit individualization and flexibility; raising basal insulin requires raising the bolus insulin and vice versa. Insulin dosage adjustments based on basal needs can impose a dietary modification to offset the increase in bolus insulin. Hence, patient education on consistency of meal timing and carbohydrate content is indicated.
Pramlintide Acetate
Pramlintide acetate (Symlin®) is a synthetic analogue of human amylin, an islet amyloid polypetide hormone secreted by the pancreatic beta cells simultaneously with insulin in response to nutrient stimuli, that slows gastric emptying, reduces postprandial rises in blood glucose concentrations and improves A1C values. The FDA has approved pramlinitide for use in patients with Type 1 and Type 2 diabetes. This non-insulin, injectable amylin analogue is administered by mealtime subcutaneous injection before meals, cannot be mixed with insulin in the same syringe and in patients with T1DM is not a replacement for but rather an addition to pre-meal insulin bolus injections.
Pramlintide regulates post-meal blood glucose levels by slowing gastric emptying and suppressing the abnormal postprandial rise of glucagon in patients with diabetes.(37) Thus, hepatic glucose production is better regulated. The effects on hepatic glucose production and increased insulin production are glucose-dependent and are overridden as serum glucose levels fall. In individuals with Type 1 diabetes, 30 to 60 mcg of pramlintide administered subcutaneously with meals resulted in sustained reductions in A1C with modest reductions in body weight.(38),(39) Hypoglycemia and nausea are the most commonly reported side effects. Nausea generally dissipates with time and can be minimized by slow upward dose titration.(38)
Medical Nutritional Therapy
Just as there is no single medical regimen that fits all patients, there is no single diet -- "there are no ADA diets" -- that can be prescribed. An individualized nutrition care program must be customized to each patient's specific preferences and needs based on a comprehensive assessment. Integrating insulin therapy with an individual's food and activity preferences is central in the management and education of patients with Type 1 diabetes. While many issues are identified during the assessment phase, glycemic control is usually addressed first, followed by nutritional interventions related to improvement of dyslipidemia and blood pressure.
As we discussed in the previous Cyberounds®, the T1DM patient must learn how to manage hypoglycemia not only for immediate safety but also because over-treating and/or fear of hypoglycemia are frequent barriers to reaching long-term glycemic goals. Some patients overeat and administer inadequate insulin in order to avoid hypoglycemia. These are not desirable strategies. While recognition and treatment of hypoglycemia are relatively straightforward, hypoglycemia prevention often requires detailed records and patient-provider teamwork. Patients need to learn the fine balance between food, exercise and medications as it relates to glycemia.
An important component of self-management is blood glucose monitoring. Choices of blood glucose monitors are plentiful; all meters are fast, and accurate when used correctly. Choosing one is based on individual preference and insurance reimbursement. For instance, a meter with a larger display may be better for elderly patients or those with visual impairment. Demonstration is effective in assessing technique and building patient confidence. The toll-free 800 number on the back of each monitor is useful for trouble shooting. Frequency and timing of testing need to be individualized according to the type of insulin regimen and changes in therapy. For instance, a patient with Type 1 diabetes who requires 4 injections or more will need to monitor at least 4 to 8 times daily, while a patient requiring basal insulin only may not need to check their blood sugar more often that once or twice daily. Target blood sugar ranges need to be reviewed and written down and patient's recordkeeping is a skill that when done properly helps to interpret insulin effects and determine therapeutic changes.
Carbohydrate Counting
Bolus dosing before meals requires the advanced skill of carbohydrate counting where carbohydrates are counted for the purpose of determining the correct pre-meal dosage of rapid-acting insulin. The carbohydrate content of food can be found on a food label, in a carbohydrate counting book or by learning that fixed serving sizes for each carbohydrate food contains 15 grams of carbohydrate. For example, 1 small piece of fruit or half a large piece of fruit each contains 15 grams.
Two principles are fundamental to carbohydrate counting. First, carbohydrate is the main nutrient that impacts blood sugar, not protein or fat.(32) A large meal of bacon and eggs has minimal carbohydrate content and, therefore, minimal impact on blood sugar. Second, the blood sugar rise is much more related to the total amount of carbohydrate than the source of carbohydrate.(32) For example, two slices of bread and six Mini Tootsie Rolls have the same carbohydrate content and, therefore, raise blood sugar similarly. Glycemic control can be achieved regardless of the amount or type of carbohydrate ingested as long as adequate insulin is administered. The most common carbohydrate counting mistakes by patients are identifying carbohydrate sources incorrectly, estimating portions incorrectly, misreading labels and snacking without administering insulin.
B is correct. As the patient's CIR is 1 unit rapid-acting insulin for every 10 grams of carbohydrate, 9 units are needed for the 90-gram breakfast. Since the patient needs to drop 40 mg/dl to reach the pre-meal target of 100 mg/dl, an additional unit is required since 1 unit drops his blood sugar by 40 mg/dl (CF = 40).
A is incorrect. Nine units may result in hyperglycemia. As the patient's CIR is 1 unit rapid-acting insulin for every 10 grams of carbohydrate, 9 units are needed for the 90-gram breakfast. Since the patient needs to drop 40 mg/dl to reach the pre-meal target of 100 mg/dl, an additional unit is required since 1 unit drops his blood sugar by 40 mg/dl (CF = 40).
C is incorrect. Eleven units may cause the patient to go low. As the patient's CIR is 1 unit rapid-acting insulin for every 10 grams of carbohydrate, 9 units are needed for the 90-gram breakfast. To correct for the high pre-meal blood sugar and reach the target of 100 mg/dl, an additional unit of insulin is needed to drop the sugar 40 m/dl (CF = 40).
D is incorrect. Cream cheese contains minimal carbohydrate. Carbohydrate is the main nutrient that impacts blood sugar, not protein or fat.
E is incorrect. The blood sugar rise is much more related to the total amount of carbohydrate than the type or source of carbohydrate. Although whole grain products are higher in nutrients and fiber, only diets with at least 50 grams (not easy to achieve) of daily fiber have been shown to significantly affect glycemia.(32)
Individuals vary considerably in the amount of insulin needed for a fixed amount of carbohydrate. A commonly used method for estimating a patient's individualized carbohydrate to insulin ratio (CIR), the amount of carbohydrate that one unit of rapid acting insulin will cover, is to divide 500 by the average number of total daily units of insulin (rapid and long-acting) administered each day.(33) A patient on an average of 30 units of insulin per day would have a CIR of about 15:1 (500 divided by 30 equals 16.6). In other words, 1 unit of rapid-acting insulin is needed for 15 grams of carbohydrate. Factors that affect insulin sensitivity can change an individual's CIR. For instance, exercise will improve insulin sensitivity. An individual with a CIR of 15:1 who anticipates eating 2 cups of rice (90 grams of carbohydrate) needs 6 units of rapid acting insulin. Once exercise is discontinued, the individual's sensitivity may decrease and the same 2 cups of rice would now require more insulin. A rise in blood sugar from a meal of more than 50 mg/dl may be a sign of either incorrect carbohydrate counting or an incorrect CIR.
In addition to the insulin dose needed to cover the carbohydrate, a supplemental dose may be required to correct for a high pre-meal blood sugar. This dose is referred to as the correction factor (CF) or the amount that one unit of insulin can be expected to decrease the level of glucose. The CF can be estimated by dividing the total number of units of daily insulin into 1500.(33) An individual with a target pre-meal blood sugar of 100 mg/dl whose CF is 40 (1 unit of rapid acting insulin drops the blood sugar 40 mg/dl) and whose pre-meal CBG is 180 mg/dl, needs an additional 2 units above and beyond the amount of insulin calculated to cover the anticipated amount of carbohydrate. For those individuals who find advanced carbohydrate counting too difficult, a fixed dose regimen (to a greater or lesser extent) can be provided. This, however, imposes the challenge of consistency in timing and carbohydrate content of meals.
Without detailed blood sugar, food, exercise and insulin-dosing records, it is difficult to identify problems and make appropriate therapeutic changes. A three-day record can provide enough of a glimpse into the complex but typically patterned lifestyle of each individual. To achieve successful treatment adherence, the health care provider must stress to their patient the importance of meaningful and engaged office visits. The rigor of intensive insulin therapy demands repeated visits by the patient and negotiations among team members. Good practice for intensive insulin therapy also requires multiple visits to a registered dietitian, preferably a CDE, during the first three months totaling 3 to 4 hours, as well as 4 to 6 additional hours of follow-up during the year. Attention to dietary management of lipids, blood pressure and weight require additional visits.
D is correct. The advantage of intensive insulin therapy using carbohydrate counting is the ability to achieve glycemic control without compromising flexibility in timing and content of meals. Glycemic control can be achieved even when skipping meals or eating more than usual. Of course, chronic overeating will result in weight gain. Patients learn to adjust their rapid acting insulin doses before meals according to the amount of carbohydrate they eat. Often, patients who carbohydrate count need to be reminded that high blood sugars from a meal are not from "too much" carbohydrate but rather arise as a result of inadequate insulin.
A is incorrect. The advantage of intensive insulin therapy using carbohydrate counting is the ability to achieve glycemic control without compromising flexibility in timing or content of meals. Patients on fixed dose regimens of rapid acting insulins need to eat fixed amounts of carbohydrate; this regimen is seldom kept by the majority for prolonged periods of time, affects quality of life and rarely achieves optimal glycemic control.
B is incorrect. It is the total amount of carbohydrate and not the source that impacts on blood sugars. As long as one counts carbohydrates and takes the corresponding amount of insulin to match the carbohydrate, glycemic control can be achieved. In practice, drinking large amounts of sugary beverages can be hard to manage due to the high caloric intake.
C is incorrect. The advantage of intensive insulin therapy using carbohydrate counting is the ability to achieve glycemic control without compromising flexibility in timing or content of meals. On the other hand, patients on fixed combinations need to eat fixed amounts of carbohydrate at regular times in order to match the peaks of the insulins injected.
E is incorrect. It is the total amount of carbohydrate and not the source of carbohydrate that impacts on blood sugars. Glycemic index of foods does not impact as much on blood sugars as the total amount of carbohydrate.
Case Report Laboratory data showed that the HbA1c improved from 11% to 7.2%. The patient returned to the nurse to review glucose records and to discuss the need for regimen changes. His records showed elevated fasting and nocturnal glucose levels. After a team discussion, it was decided to perform a 72-hour continuous glucose monitoring sensor test to better identify glucose trends and make more effective therapeutic choices. The team strongly reminded the patient that tight glycemic control (an HbA1c close to 6%) is paramount if the patient wants to avoid micro and macrovascular complications. |
Continuous Glucose Monitoring Sensor (CGMS)
CGMS is used when more intensive monitoring is required. A glucose sensor, placed under the abdominal skin, measures interstitial glucose every 10 seconds and these glucose values are averaged every five minutes. These measurements are highly correlated with finger stick values.(34) The results are downloaded to a computer where graphs and tables reveal glucose trends. This device can be especially helpful in complicated situations such as hypoglycemia unawareness, gastroparesis, gestational diabetes, preconception, pregnancy and lactation where the patient and doctor want to achieve tighter control. More recently, Medtronic MiniMed has received approval for a patient version of the CGMS, the Guardian RT. This system displays real time glucose values every 5 minutes and sounds an alarm or vibrates if glucose levels go too high or to low. The Guardian RT is approved for patients 18 years of age or older who have Type 1 or Type 2 diabetes. By obtaining frequent glucose values, individuals can more readily see the effect of diet, exercise and medications and make the needed changes that will result in fewer glucose excursions. In this case, CGMS may help to assess the effectiveness of basal and bolus insulins.
A is correct. The mean blood glucose concentration obtained postprandially (1 to 2 hours after a meal) primarily reflects the effectiveness of the bolus insulin dose. Postprandial values indicate if adequate bolus insulin was given to match the amount of carbohydrate consumed.
B is incorrect. The mean blood glucose concentration before meals (before breakfast, before lunch and before dinner) primarily reflects the effectiveness of the basal insulin.
C is incorrect. A nocturnal test reflects the effectiveness of the basal insulin overnight. It is common to see a sharp rise in blood glucose beginning at 2:00-3:00 AM from the dawn phenomenon.
D is incorrect. The fasting blood glucose level reflects the adequacy of the overnight basal insulin.
E is incorrect. The blood glucose level post exercise may reflect either basal or bolus excess, depending upon when the exercise took place. Generally, if the activity takes place within three hours after eating, a 50% reduction of mealtime insulin should be recommended. If the exercise takes place before the meal or lasts longer than 30 minutes, a reduction in basal insulin for 24-48 hours may be necessary, as glucose levels may be affected up to 48 hours later. To prevent hypoglycemia, extra carbohydrate calories may also be necessary prior to exercising.
Case Report, Continued Three days later, the sensor was downloaded and showed consistent elevated glucose levels between 3:00 AM and mid-morning. Two possible regimen changes were discussed:
The patient elected to continue multiple daily injections. In an attempt to further improve his glycemic control, he asked about adding Symlin to his current regimen. It was explained that although Symlin improved hyperglycemia, it was not realistic at this time, as it would mean at least 8 injections daily. The team promised to evaluate Symlin in the future once a repeat A1C is obtained and/or if the patient decides to change to the insulin pump (continuous subcutaneous insulin infusion). |
Continuous Subcutaneous Insulin Infusion (CSII)
CSII is a method of delivering insulin through a motor driven reservoir into a self-inserted subcutaneous infusion set; the insulin serves as both basal and bolus. All rapid-acting insulin analogues are more effective than regular insulin in insulin pumps -- they achieve better glycemic reductions in postprandial blood glucose concentrations and provoke fewer episodes of hypoglycemia compared with regular insulin.(35),(36) The basal insulin consists typically of 40-60% of the total daily dose and is individually programmed and adjusted by 0.05 units every 30 minutes to match the changes in basal needs throughout the 24-hour day. Insulin delivered via CSII is absorbed better and more predictably than the "depot" of insulin delivered in multiple daily injections (MDI).
The pre-meal bolus insulin amount can be automatically calculated by programming the pump for the patient with pertinent figures including the carbohydrate to insulin ratio, correction factor, glycemic targets and duration of insulin action. The patient enters the anticipated carbohydrate amount and blood sugar. The calculation takes into account the active insulin on board, subtracting this insulin from the estimated correction dose, thereby avoiding stacking of insulin and hypoglycemia. Pump features that promote individualization include bolus delivery over an extended period to match prolonged digestion and absorption of food. For example, a high fat meal treated with an injection of rapid-acting insulin often results in an initial hypoglycemic response followed by hyperglycemia when the food finally is finally absorbed.
Studies comparing MDI to CSII demonstrate comparable HbA1C values, though patients on CSII report less hypoglycemia, post-meal glucose excursions and better quality of life.(36) Some disadvantages of the insulin pump are the higher cost for the pump and its supplies, increased skin infections at the insertion site and greater chance of mechanical problems such as interruption of insulin flow or pump failure that can lead to diabetic ketoacidosis (DKA). An early malfunction that is not recognized can lead to DKA within 4 to 6 hours. Therefore, it is imperative for all individuals using an insulin pump to test blood glucose levels frequently (no less then 4-6 times each day), be properly trained in the use of the pump and learn how to avoid and treat acute complications proactively. It is important that those who choose the pump as a delivery system clearly understand that the convenience of no injections is offset by many other responsibilities and that the pump is a delivery system that is only as effective as its user. A surgically-implanted programmable insulin pump, which delivers insulin directly into the peritoneal cavity, is under investigation. At this time, technical problems have been a barrier to approval.
Summary
This is a case of a 51-year-old male who developed Type 1 diabetes also known as Latent Autoimmune Diabetes of the Adult (LADA). In the first Cyberounds on this case we reviewed how to establish a correct diagnosis, the essentials of patient education and the importance of early and aggressive treatment. In Part 2, we discussed intensive insulin management using a team approach. The prescription of an effective basal-bolus regimen is intricate and requires a team effort that includes the patient, nurse, dietitian, endocrinologist and others. This labor-intensive endeavor needs to be individualized through trial and error. A variety of insulins including the more recent analogues enable better physiologic insulin replacement treatment, with the majority of patients being treated with a basal-bolus regimen. Proper adjustments of bolus insulin need to be done according to carbohydrate counting and the constant variations in insulin sensitivity.
Recently, the FDA has approved an injectable non-insulin amylin analogue and a non-injectable inhaled insulin. The wide choice of new medications and gadgetry include pen syringes, insulin pumps and better systems for monitoring, all of which have made management of Type 1 diabetes easier. These advances have improved insulin administration and adherence, reduced episodes of hypoglycemia and fostered a better quality of life for patients but they have only had a modest impact on glycemic control. The Continuous Glucose Monitoring Sensor (CGMS) is slowly evolving into a closed loop system where glucose sensors will provide feedback to implantable insulin pumps so as to deliver insulin requirements more precisely. It must always be remembered, however, that successful outcomes can only be achieved when effective team management and education are provided in combination with a patient who is proactively involved in self-management.