Course Authors
Liliane Min, M.D., Peter Barland, M.D., and Elena Peeva, M.D.
Dr. Min is a Fellow in Rheumatology and Dr. Peeva is Assistant Professor of Medicine, both at The Albert Einstein College of Medicine, New York.
Within the past 12 months, Dr. Peeva has received grant support from Immunomedics. Drs. Min and Barland report no commerical conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the B cell-depleting therapies for SLE now undergoing clinical trials
Discuss the B cell-stimulating cytokines for SLE now undergoing clinical trials
Discuss the safety profile of the above treatments.
Dr. Peeva and colleagues will discuss the unlabeled use of rituximab, epratuzumab and belimumab for the treatment of systemic lupus erythematosus.
SLE is a chronic autoimmune disease of unknown etiology. It is characterized by dysregulation of multiple aspects of the immune system leading to B cell hyperactivity and production of variety of autoantibodies. The current treatments for SLE are non-specific and include non-steroidal anti-inflammatory drugs, anti-malarials, steroids and cytotoxic agents. These therapies, especially the high dose steroids and cytotoxic drugs, have multiple side effects including infections which may be life threatening. In addition, a significant number of SLE patients do not respond or show only partial response to the currently approved therapies. Thus, the current therapeutic arsenal for SLE is inadequate and more specific treatments are desperately needed.
Given the central role of B cells in the pathogenesis of SLE, B cell-targeted therapies are of special interest in the treatment of SLE. As discussed previously, B cells have multiple roles in the adoptive immune response. Mechanisms of B cell depletion and inhibition, using monoclonal antibodies to specific cell surface markers, as well as the blockade of B cell-stimulating cytokines, are of clinical importance. In this Cyberounds®, we focus on B cell-targeted therapies that are currently in clinical trials for SLE.
B Cell-Depleting Therapies
Two monoclonal antibodies that target B cell surface antigens CD20 and CD22 are currently undergoing clinical trials in SLE. Rituximab, a chimeric mouse-human mAb targeting the CD20 antigen on B cells was initially studied in the treatment of refractory low grade or follicular B cell Non-Hodgkin's lymphoma (NHL).
Figure 1. CD20.
|
 |
Johnson P et al. Semin Oncol. 2003; 30:3-8; Golay J et al. Blood. 2000; 95:3900-3908.
In the field of autoimmune diseases, rituximab has been most extensively studied in RA, where a large randomized double blind controlled study has recently been published.(1) Similarly, epratuzumab, an anti-CD22 humanized mAb, is undergoing phase II/III clinical trials in refractory NHL, as well as in autoimmune diseases such as SLE.(2)
Rituximab and epratuzumab deplete B cells that express the CD20 or CD22 antigen respectively but do not affect plasma cells because plasma cells do not express either antigen on their surface (see Figure 2).
Figure 2. B Cell Membrane Markers.
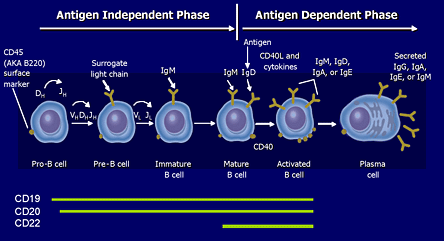
Adapted from Sell S et al. Immunology, Immunopathology, and Immunity. 6th ed. Washington, D.C.: ASM Press; 2001. Roitt I et al. Immunology. 6th ed. Philadelphia, PA: Mosby; 2001. Tedder TF et al. J Immunol. 1985; 135:973-979.
CD20 (and CD19) are membrane markers found on the surface of B cells during a defined portion of their maturation.
The CD20 antigen is expressed on B cells in all stages of their development, from early pre-B cell stage to early plasmablasts, and is extinguished upon terminal differentiation into mature plasma cells. Therefore, rituximab can potentially deplete peripheral B cell counts below detectable limits. In contrast, CD22 expression on B cells is more limited, appearing on the cell surface of IgM+IgD+ mature B cells and disappearing with B cell differentiation into plasma cells. Thus, anti-CD22 antibodies do not cause full B cell depletion but rather a decline in B cell levels ranging from 40-60%. In addition, anti-CD22 therapy may negatively regulate hyperactive B cells through its inhibitory effect on the B cell receptor (BCR).(3),(4)
B Cell-Depleting Therapies in Systemic Lupus Erythematosus -- Preliminary Results
Rituximab
In 2002, Leandro and colleagues reported preliminary evidence for the safety and efficacy of B lymphocyte depletion therapy in SLE patients.(5) Six female patients were treated in an open label trial with a combination of rituximab, cyclophosphamide and prednisolone. Patients were given two 500 mg infusions of rituximab, two 750 mg infusions of cyclophosphamide and oral prednisolone (30 or 60 mg daily for five days), two weeks apart. One patient was lost to follow-up after three months, while the remaining five patients showed an improvement in disease activity as measured by the British Isles Lupus Assessment Group (BILAG) scores at three and six months post-treatment. Mean BILAG scores improved from a median of 14 (baseline) to a score of 6 (at six months).
Clinical manifestations of serositis, arthralgia/arthritis, fatigue and skin vasculitis responded particularly well. Three patients had diffuse proliferative glomerulonephritis (DPGN), with two of these patients showing a decrease in proteinuria, while the rest of the patients remained stable at six months. Improvements in ESR, hemoglobin and C3 levels were noted with no significant change in anti-dsDNA titers. Total IgG and IgM levels remained within normal range. B lymphocyte depletion (less than 5 cells/ml) was seen in all patients and lasted for a mean of four months; one patient remained B cell depleted for more than 16 months of follow-up. Clinical remission lasted an average of 12.6 months, with two patients relapsing after seven to eight months. There were no adverse events noted during the infusions. Nine infectious episodes were reported but were self-limited and responded well to antibiotics.
The same group recently reported the results of 21 SLE patients treated with rituximab in the past four years, which included the six patients reported above.(6) The patients were refractory to immunosuppressants including cyclophophamide and mycophenolate mofetil. Six patients were treated with low dose rituximab (500 mg IV x 2 doses) and 13 patients were treated with high dose rituximab (1000 mg IV x 2 doses) with concomitant cyclophosphamide therapy. At a mean follow-up of 19 months, clinical parameters such as fatigue, arthritis/arthalgia, serositis, nephritis, thrombocytopenia and hemolytic anemia improved. Mean B lymphocyte depletion lasted between three and eight months, with the exception of one patient, who did not deplete well secondary to forming human anti-chimeric antibodies (HACA). During nine months of follow-up, no serious infections developed.
Looney and colleagues published a phase I/II dose-escalation trial of rituximab added to concomitant therapy in 18 patients with SLE.(7) Rituximab was administered as a single infusion of 100 mg/m2 or 375 mg/m2 to 12 patients and as four infusions of 375 mg/m2 one week apart in six patients. One patient withdrew prior to receiving any infusion but of the 17 remaining patients 11 patients achieved B cell depletion. In these patients, clinical efficacy, as measured by the Systemic Lupus Activity Measure (SLAM) score, was significantly improved at two and three months, with improvement persisting for 12 months. Clinical response was specifically notable for rashes, mucositis, alopecia and arthritis. During the study, serum anti-dsDNA antibodies, C3 and C4 levels did not change significantly in the entire group of patients. Poor B cell depleters did not show any significant decrease in disease activity.
The cohort of patients included seven patients with lupus nephritis (class III or IV). One patient experienced improvement in his class IV nephritis, with resolution of hematuria, proteinuria and normalization of renal function and serologies. On repeat renal biopsy 12 months after the therapy, there was a complete resolution of the proliferative changes. Among all patients treated, only one patient had a mild infusion reaction which did not require withdrawal from the study. No deaths, malignancies or opportunistic infections were observed during the 12 months of follow-up. Mean IgG and IgM levels decreased at six and nine months but were not associated with infections. There was no decrease in levels of antipneumococcal and antitetanus titers. Of the 17 treated patients, 11 developed HACAs, and six of them had high HACA titers. HACAs were typically detected two months post treatment and were associated with less B cell depletion, higher baseline SLAM scores and African-American ancestry.
Albert and colleagues have also conducted a pilot, phase I study using rituximab in 11 patients who failed previous immunosuppressive therapy.(8) All patients received four weekly infusions of rituximab at 375 mg/m2, as well as methylprednisolone 100 mg IV prior to each infusion. Patients were withdrawn from previous immunosuppressive therapy but remained on stable doses of oral steroids. Eight patients completed all four infusions -- six of these patients demonstrated 99% B cell depletion and significant clinical response, as measured by the SLE Disease Activity Index (SLEDAI). Clinical remission was long-term in two out of the six patients, lasting 6-14 months, and allowed reduction of oral steroid therapy. Four out of six patients had short-term remission, lasting four weeks to six months, and required retreatment with another immunosuppressant but had overall better disease control. Serum C3, C4, anti-dsDNA antibody titers and immunoglobulins level did not change during the study. Overall, the safety profile of rituximab was acceptable.
A recent uncontrolled clinical trial showed that rituximab may also have a role in the treatment of neuropsychiatric lupus,(9) which is one of the most serious and treatment-resistant clinical presentations of SLE. Twenty-two patients with neuropsychiatric SLE were included in the study; 12 patients were treated with rituximab only (1 g), seven patients were treated with rituximab and cyclophosphamide (750 mg) and three patients were treated with plasmapheresis and cyclophosphamide followed by rituximab. The patients were followed regularly over the next year and a half and at the 18-month follow-up, 14 out of 19 patients treated with rituximab showed significant improvement. In this study, as in the ones mentioned above, rituximab was well tolerated.
Epratuzumab
In contrast to rituximab, there has been only one completed study with epratuzumab in SLE. In an open label clinical trial, Kaufmann and colleagues evaluated epratuzumab in patients with SLE(10) to assess feasibility, safety and early evidence of efficacy.(11) Fourteen patients with SLE with current disease activity of 6-12, as determined by BILAG scores, were given four doses of intravenous epratuzumab at 360 mg/m2 two weeks apart. The BILAG scores improved in all patients, with 73% achieving greater than 50% improvement from the baseline score.
In all patients, clinical improvement was maintained at 10 weeks and in 78% of patients at 18 weeks. B cell numbers declined 60% at four weeks and 40% at six months. As expected, no changes were noted in the serum levels of autoimmune antibodies and immunoglobulins. Also, no evidence of human anti-epratuzumab antibodies was detected. Infusions were well tolerated with premedication with Tylenol and Benadryl. Two patients developed mild infections (herpes zoster and otitis media), which responded well to antivirals/antibiotics.
Anti-cytokine Therapy
BlyS (also known as BAFF) is a secreted cytokine of the TNF family that exerts its effects by three different types of B cell membrane receptors (BCMA, BAFFR and TACI).(12) BlyS stimulates B cell survival, development and differentiation into plasma cells. BlyS levels are elevated in lupus-prone mice, and administration of soluble Blys receptors ameliorates disease progression and improves survival of these mice. SLE patients also have elevated BLyS levels and increased BlyS correlates with autoantibody titers.(13),(14)
Belimumab is a fully human mAb that binds and neutralizes soluble human Blys. Furie and colleagues have conducted a phase I, double-blind, randomized study in patients with SLE, evaluating the safety, tolerability and immunogenicity of belimumab.(15) Four different infusion doses (1, 4, 10, 20 mg/kg) of belimumab or placebo were given as a single infusion or two infusions administered 21 days apart since the half-life of the agent is 13-17 days. Seventy SLE patients with stable or mild-moderately active disease were enrolled in the study, 13 patients in the placebo arm and 57 in the treatment arm.
Belimumab-treated patients had significant reductions of CD20 positive B cells (ranging from 12-47%). During 42-105-day follow-up, no change in SLE activity was noted. Belimumab was well tolerated; no increased incidence of adverse events, including infections, was observed. One patient developed antibodies to the agent but no adverse events secondary to the formation of these antibodies occurred.
A Phase II, double-blind, placebo-controlled trial on safety and efficacy of three different doses of belimumab is underway. Preliminary results have failed to show the efficacy of this agent in reducing signs and symptoms of SLE after 24 weeks, or reducing the time to first flare over a 52-week treatment period. However, conclusions cannot be drawn since the final results are not yet available for interpretation.
Safety Profile
The most common adverse events during or following infusion therapy with both rituximab and epratuzumab are mild to moderate in severity, consisting of fevers, chills, rigors, nausea and, less commonly, hypotension and dyspnea.(2),(16) Experience in oncology patients, where infusion reactions are more frequent, shows that premedication with antihistamines, acetaminophen, or co-administration of steroids or slowing the infusion rate are quite effective in preventing or treating these adverse events. The incidence of an infusion reaction is more common with the first infusion than with the subsequent ones. In summary, few serious adverse reactions with rituxan were reported.
Figure 3. Detailed SAEs in Rituximab Therapy.
| MTX (n = 40) | RTX (n = 40) | RTX + CTX (n = 41) | RTX + CTX (n = 40) | |
| Corneal abscess | 1 | |||
| Pneumonia (pseudomonal) | 1 | |||
| Bronchopneumonia* | 1 | |||
| Septic arthritis | 1 | 1 | ||
| Septicemia | 1 | |||
| Tendon rupture | 2 | |||
| Lumbar vertebral fracture | 1 | |||
| Anemia | 1 | |||
| Renal impairment | 2 | |||
| Pregnancy/abortion | 1 | |||
| Thrombosis | 1 | |||
| Pericarditis | 1 | |||
| Gastroenteritis (viral) | 1 | |||
| Goiter | 1 | |||
| Arytenoiditis | 1 | |||
| Migration of renal stent | 1 | |||
| Total | 3 | 4 | 8 | 4 |
*Preliminary data.
1-24 weeks; 25-48 weeks.
* Patient death
Szczepaski L et al. 67th Annual Meeting of the American College of Rheumatology, October 23-26, 2003; Orlando, Florida.
Figure 3 details all serious adverse reactions in the different treatment groups.
Antibodies against rituximab have been detected in two SLE trials.(7),(8) Looney and colleagues indicated that 6 out of 17 patients developed high HACA titers,(7) a rate significantly higher than the incidence of these antibodies in lymphoma patients. Serum sickness with high HACA titers has been reported in patients treated with rituximab for IgM-associated neuropathy, ITP and Sjogren's Syndrome.(17),(18),(19),(20)
Studies using epratuzumab in NHL show a low incidence of human anti-human antibody (HAHA), with only 2% of all patients testing borderline positive and with no associated clinical events. In the study by Kauffman in SLE patients,(2) no HAHA were detected. Further long-term data is needed before conclusions can be made about the clinical significance of the antibodies induced by rituximab and epratuzumab in SLE patients.
Follow-up in open label trials involving patients with SLE has not shown increased risk of serious infections, opportunistic infections or malignancies with the administration of rituximab or epratuzumab. Also, data from a randomized, double-blind, placebo-controlled trial of rituximab for treatment of refractory RA showed no increased rate of infections with the drug, with follow-up available 48 weeks post treatment.(21) However, increased risk of infections are of concern in children, where rituximab has been shown to significantly lower the immunoglobulin levels with resulting serious infection.(20),(22) Therefore, intravenous immunoglobulin is often used prophylactically in infants and in older children when IgG levels fall below normal. The same prophylaxis and treatment may be warranted if rituximab is used to treat pediatric patients with SLE.
Belimubab has demonstrated a good safety profile in both phase I and phase II clinical trials in RA. The current data from the SLE clinical trials with this agent have not shown any serious adverse events, including infections.
Conclusions
B cells play a crucial role in the pathogenesis of SLE. They are antibody and cytokine producing cells, as well as important T cell activators, and alterations of any of these immune functions may be implicated in the development and/or flares of lupus. Our better understanding of the B cell biology has led to the development of novel therapeutic approaches in the treatment of lupus that specifically target B cells. Several clinical trials of B cell depleting therapies, as well as of agents that block cytokines that stimulate B cells, are underway.
The phase I and II SLE clinical trials with agents that specifically deplete B cells have provided encouraging results proving that B cells play a key role in continued activity of established disease. Clinical improvement seen in these studies has not clearly been associated with decrease in autoantibody levels, suggesting that B cell depletion generates tolerance through a variety of mechanisms, such as preventing activation of autoreactive T cells and generation of proinflammatory cytokines.
For the treatment of refractory SLE, the use of B cell depletion, either by itself or with concomitant immunosuppressive therapy, seems to be efficacious with an acceptable safety profile. Questions still remain about the optimal dosage and timing of infusions, and whether concomitant immunosuppressive therapy is needed to provide optimal clinical response and prevent formation of HACAs. Hopefully, the current phase III clinical trials with rituximab and epratuzumab will answer these questions in the near feature.
Belimubab is an mAb that blocks B cell stimulating cytokine BLyS. The tolerability and safety of this mAb seem good but its efficacy is less established. The preliminary results of the phase II clinical trial failed to demonstrate that the medication is achieving the primary and secondary endpoints. However, final results are needed before conclusions can be made about belimubab in treatment of SLE.