Course Authors
Susan C. Stewart, M.D.
This activity is made possible by an unrestricted educational grant from Roche Diagnostics. ![]()
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe current methods of measuring bone mineral density and how to interpret the results
Detect risk factors and physical signs of osteoporosis when evaluating patients
List and compare the current therapies for osteoporosis.
I would like to thank Dr. Catherine Hammett-Stabler, Associate Professor, Department of Pathology and Laboratory Medicine, University of North Carolina-Chapel Hill, and Associate Director of Core Laboratory (Director: Clinical Toxicology, Clinical Pharmacology, Endocrinology, Pediatric Metabolism, Special Chemistry) McLendon Laboratories, UNC Hospitals, Chapel Hill, NC, for her helpful information and advice for this Cyberounds®.
The two most devastating effects of osteoporosis (OP), hip fracture and vertebral fracture, stand out as a testament to the effects of increased life span and altered lifestyle in our modern age. In the United States, poor nutrition, or wrong nutrition, and inadequate activity compound the already present genetic predisposition that many in the population have for fragile bones. Counteracting that, of course, is our obesity epidemic. Though the extra weight of obesity may indeed strengthen bones, an obese individual will likely contend with arthritis and obesity related illnesses.
Previously, clinicians needed to wait for a fragility fracture to enable us to diagnose osteoporosis but now we have bone density tests and many treatments for osteoporosis. Primary osteoporosis is age-related bone loss, resulting in fragile bones at increased risk for fracture. Secondary osteoporosis is bone loss caused by medication, malignancy, endocrine, renal, gastrointestinal and other systemic diseases.
Bone Fragility
Although we now identify osteoporosis with a lab result measuring bone density, it is probably more useful to think of the disease as the manifestation of bone fragility. This gives us a chance to examine the processes by which an imbalance between bone formation and bone resorption tips toward net bone loss during the lifetimes of men and women.(1)
In bone tissue, the helical structures of type I collagen are stiffened with mineral crystals of calcium hydroxyapatite. This structure confers strength and flexibility, so that bones can support the weight of the body but have "give" to accommodate muscle contraction and compressive loading. Bone is an active metabolic tissue and is constantly being formed by the osteoblasts and resorbed by osteoclasts.
About 10% of bone is replaced each year, so that the entire skeleton turns over every 10 years. This metabolic activity occurs throughout the different types of bone tissues, the cortical and cancellous. Cortical bone comprises the thick shaft of bone in the long bones and the outer shell of bone on the vertebral bodies. Cancellous bone is the more spongy appearing structure, for example, the inner portion of the vertebral bodies or the ends of the long bones. The sites of metabolic activity are the periosteal, or outer surface of the cortical layer, and the endosteal, the inner surface adjacent to and within the marrow cavity. The endosteal sites of metabolic activity are the intracortical, the endocortical and the trabecular. The intracortical is within the cortical layer of bone; the endocortical is the inner surface of the cortical bone; the trabecular is the network of interconnected shafts that make up cancellous-type bone.
Bone Formation and Bone Resorption
I dwell on these types of surfaces for metabolic activity to sensitize you to the concept that there are many sites for formation and breakdown of bone. Also these sites vary in their sensitivity to hormonal action. Androgens stimulate periosteal bone formation; estrogens inhibit it. Higher levels of estrogens stimulate more endosteal bone formation in women. In the long bones, the actual cortical thickness may be the same in men and women but because of their greater diameter, bones in males are stronger in resisting stress. Estrogens are active in bone formation in men as well as women, and the net thickness of trabeculae in cancellous bone will be greater in men. Because of the larger diameter of cortical bones and thicker trabeculae of cancellous bones, men have a larger bone mass than women.
Estrogens not only increase bone formation, they inhibit bone resorption by decreasing the activity of osteoclasts. When estrogen is withdrawn, bone resorption increases. The actual mechanism is a decrease in the inhibition of osteoclast formation. The increase in the multicellular osteoclasts, and the larger number of resorptive sites they occupy, creates an imbalance between formation and breakdown of bone, resulting in net bone loss. In women this is accelerated at the time of menopause with the steep drop in estrogen.
In the first 5-8 years of menopause, 1%-2% of bone is lost per year in addition to the 0.4% of bone lost per year after peak bone density is achieved at age 30.(23) A new steady state is reached as osteoblasts form bone in the cavities made by the osteoclasts. Since osteoblasts lay down matrix first and then the minerals, there is a lag in which bone mineral density tests plummet rapidly at menopause and then level down to a slower rate of loss as mineralization occurs. Another effect of absent estrogen is an increase in the lifespan of osteoclasts and a decrease in the lifespan of the bone-forming osteoblasts. In men, age-related decreases in estrogen also result in increased bone resorption.
When estrogen is decreased, more precursor cells in the bone marrow differentiate into osteoclasts. These multinuclear cells gouge holes, called lacunae, into bone tissue. The effect is particularly devastating in cancellous bone. Think of the trabeculae as a three-dimensional lattice of crisscrossing pillars of bone tissue. The osteoclasts not only dig out lacunae, in a thinner trabecula the osteoclasts may actually sever it completely, resulting in a loss of connectivity of the lattice structure. This occurs more frequently in women, who have thinner trabeculae to begin with. Vertebrae with severe loss of connectivity are at high risk for collapse, and multiple vertebral fractures result in kyphosis, the so-called "dowager's hump" seen in so many older women.
Research on osteoclast inhibition has now led to the discovery of the osteoclast differentiation factor, called receptor activator of nuclear factor-kappa B ligand, or RANKL. This ligand is the common factor for all mediators of osteoclast formation. Compounds that inhibit RANKL would decrease osteoclast formation and tip the metabolic balance toward bone formation, opening new opportunities for treatment.
Measuring Bone Mass
The term "bone mass" simply means the amount of bone in the whole body or in a specified area of bone. We currently measure bone mineral density (BMD) and use that as a surrogate for bone mass. From BMD measurements we have developed standards to determine the presence of osteoporosis. The techniques used include single and dual-energy x-ray absorptiometry (SXA & DXA), quantitative computed tomography (QCT) and quantitative ultrasound.
In SXA and DXA, bone mineral density is expressed as the mineral content divided by a given area of bone in two dimensions. A larger bone will yield a higher BMD (because the X-ray traverses a larger area of bone and, therefore, "sees" higher density), even though the volumetric bone density, i.e., the mineral content per cubic centimeter, may be similar in each bone.(2) The sites usually measured are the hip and lumbar vertebrae. DXA is currently the test used most commonly for diagnosis and treatment decisions for osteoporosis.
QCT measures density in three dimensions and can give a more accurate picture of trabecular bone density. The radiation exposure is much higher than DXA and its value in clinical practice has not been determined.
Ultrasound can determine bone density based on the transmission of sound waves through bone. This has been used on the heel bone as a screening test. DXA and SXA can also measure BMD at the heel or the distal radius. These peripheral measurements are less expensive than hip and spine DXA. There is more variation in the results compared to DXA. They are best used for screening and referral for definitive diagnosis and treatment if indicated.(2)
Recommendations and Risk Factors
In 2002, the U.S Preventive Services Task Force issued a guideline that all women over age 65 be screened for osteoporosis. They based this guideline on evidence that BMD measurements accurately predict the risk for fracture and that treatment of asymptomatic women with osteoporosis reduces the risk for fracture in the future. They also recommended screening 60-year-old women who have risk factors for osteoporosis. The risk factors to be considered are low body weight, weight loss, family history, smoking, alcohol or caffeine use, low calcium and vitamin D intake.(3),(5) (http://www.ahrq.gov/clinic/uspstf/uspsoste.htm) The National Osteoporosis Foundation also recommends screening postmenopausal women who have had a fracture.(3)
We clinicians have been criticized for not following these guidelines and adequately treating patients (men and women) found to have osteoporosis. In this section, I will make some suggestions about questions and maneuvers you can incorporate into your history and physical exam that might alert you to order a bone density test on a patient.(6)
History and Physical Exam
Chief Complaint: The patient says her (his) back is becoming humped. A patient who notices a back deformity has a strong chance of having osteoporosis of the spine.
Fracture: wrist or other fracture not due to major trauma in a postmenopausal patient.
Family History: As mentioned before, there are strong genetic determinants of bone density, so a family history of osteoporosis, particularly as manifested by a maternal hip fracture, should alert you to increased risk in your patient. There are racial differences in bone density. Asians have a lower bone density than whites. On the other hand, African-Americans have higher bone density.(1)
Surgery: Hysterectomy and oophorectomy, especially earlier than average menopausal age. Gastrectomy will interfere with calcium absorption.
Habits: Both cigarette smoking and excessive alcohol are positively associated with the development of osteoporosis.
Exercise: Lack of weight-bearing activity, particularly if combined with low weight. Conversely, excessive exercise combined with very low body fat and amenorrhea.(7)
Diet: Low calcium and vitamin D intake. Eating disorder.(7)
Medications: Corticosteroids, anticonvulsants, heparin, cyclosporine, aromatase inhibitors. Medication for hyperthyroidism.(6),(22)
ROS: Malignancy of the bone or marrow; chemotherapy, especially leading to premature menopause or gonadal insufficiency.
GI symptoms: Diarrhea may indicate celiac disease or lactose intolerance.
Musculoskeletal: Back pain. Weakness. Falls. A fracture, especially from low trauma, and especially after age 50.
Physical Exam
Physical measurements: Standard weight and height and a few physical maneuvers can alert you to the need to screen for osteoporosis.
Weight of less than 60 kg or 132 lb has a positive likelihood ratio (LR+) for OP of 3.6, and weight less than 52 kg or 112 lb carries an LR+ of 7.3.(6)
Height: especially a decrease from a previous level, either recalled or documented by previous physical measurement.
Two simple tests for physical evidence of vertebral fractures are:
- Wall-Occiput Distance: Inability to touch occiput to the wall when standing with back and heels against the wall;
- Rib-Pelvis Distance: With the patient standing and the arms outstretched parallel to the ground, measure the distance between the lower border of the rib cage and the pelvic rim in the mid-axillary line. A less than two fingerbreadths distance should raise your suspicion for vertebral fracture.(6)>
A good screening test for motor strength, coordination and balance is the "Get-Up and Go" test. Ask the patient to rise from a chair walk a few yards, turn abruptly and walk back. Using the arms to rise from the chair denotes diminishing motor strength, as does a slow speed of walking. The abrupt turn tests balance and coordination.(8)
Head and neck: Check the teeth. A tooth count of fewer than 20 teeth reflects a significant loss of bone supporting the teeth and is a clue to underlying osteoporosis.(6)
Neurological: If you have a concern about balance, check vibration sense. It is a more accurate test of proprioception than position sense.(8)
Using Bone Mineral Density Tests for Diagnosis and Therapy
You order the test because you followed the guidelines or because of raised suspicion based on your history and physical exam findings.
You have the result, now what does it mean? Most standard DXA tests are of the hip (total and sometimes specific areas like the femoral neck, trochanter, etc.) and the spine, L1 to L3 or L4, listing the values for each vertebra and the total spine. The test results usually come with an alarming display of columns of numbers, percentages, scores and a graph. The graph plots the values and standard deviations of BMD against age. The standards and deviations are drawn from large populations and must be of the same sex and race as your patient. The norms for a population of African-American men or women will be different from those of white men or women. On the graph will be an "x" showing you where your patient's value falls. If you get both hip and spine, you will get a different graph with numeric values for each site.
The T and the Z scores: These scores are reported as percentages of two different norms. The T score compares your patient with a population of young adults of the same race and sex as your patient at the time of attainment of peak bone mass for that group, generally around age 30. The Z score compares your patient to a population of adults of the same age. Since adults have steadily decreasing norms of BMD after the third decade, you can see that the Z score for a 65-year-old may be quite normal compared to other 65-year-olds, but when you look at her T score you will find it to be much lower, because it is now being compared to 25-45 year-olds. These scores are expressed as percentages of the norm. We look at the T scores because that compares our patient with the theoretical peak bone density of the young adult.

Figure 1. Changes in Bone Mineral Density Over Time.
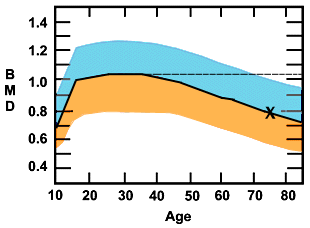
The ordinate is the bone mineral density and the abscissa is age in years. The blue portion is two standard deviations above, and the orange is 2 SDs below the median in a population of white women. Note the sharp upsurge in the teenage years, the leveling off and peaking around age 30, and the gradual decline that increases at age 50, the time of menopause. The dotted line extending from the 30-year-old median peak, is the T score line. The "x" marks the BMD measurement of a 75-year-old woman at the median for her age. Her Z score would be 100% but her T score would be osteoporotic, 2.5 standard deviations below the young adult peak.

The World Health Organization has determined that a T score of minus 2.5 will define osteoporosis.(4) That's 2.5 standard deviations below the young adult norm for the sex and race of your patient. This level has been set to enable bone density comparisons between populations and is not meant to dictate treatment. However, T scores in this range do define a population at risk for fracture that has been shown to benefit from treatment. Osteopenia has been defined as a BMD of -1.0 to -2.5. The risks associated with this level and the benefits of treatment are less clear.(9)
Special Notes About Interpretation of BMD Values
Different machines may use different populations, even of the same race and sex, for their standards, so if you are following a patient with periodic BMD tests you must use the same type of test and the same type of machine for serial measurements.
What to do if spine and hip values differ, with one above and one below -2.5: A number of experts and guidelines consider that a hip (total or femoral neck) score of -2.5 or less is most specific for osteoporosis and defines a population with the highest risk for fracture by BMD criteria. If you have to choose, use the hip.(6)
Will arthritic changes in the spine change BMD values? Yes. One problem with spine BMD measurements in the older population is that calcium deposits in arthritic facet joints will be picked up and give a falsely higher measurement by DXA. The actual density of the trabecular bone in the vertebral body may be quite low. There may be different readings for different vertebrae. In the presence of arthritis, use the lowest reading as your criterion. The lateral chest X-ray can detect arthritic changes and significant mineral loss in vertebral body cortical and trabecular bone.
Looking for secondary osteoporosis: Medical conditions like hyperthyroidism, hyperparathyroidism, Cushing's disease, and many others, can cause osteoporosis but routine screening for these conditions is probably not cost effective. Some authors think that it is more useful to look at the BMD compared to the age group, the Z score rather than the T score. A Z score of -2 to -3 may indicate an underlying medical illness or effect of chronic medication that should be detected. The underlying condition must also be treated in order to insure success with specific treatment for osteoporosis.(6)
When are X-rays helpful? Standard radiographs are highly insensitive indicators of bone loss and are not used for screening. However, a report of bone demineralization or a vertebral fracture on a chest radiograph should trigger a bone density test. A positive occiput to wall or rib-pelvis distance test requires a lateral chest x-ray looking for vertebral fracture. A woman with a vertebral fracture has a four-fold increased risk of another vertebral fracture and that risk will be decreased by treatment.(6)
Bone Markers
A number of substances are released into the circulation during the processes of bone resorption and formation that can be measured and used to mark the progress of treatment. Because there are so many variables contributing to the values of these markers, they are not used for diagnosis. Some of the markers currently measured are:
Resorption markers: When type-I collagen is broken down by the osteoclasts, the N-terminal telopeptides, abbreviated NTx, and the C-terminal telopeptides, CTx, and ring structures called pyridinium crosslinks are released. There is also tartrate resistant acid phosphatase.(22)
Formation markers: During bone matrix formation the osteoblasts secrete a noncollagenous protein called osteocalcin and type-I procollagen peptides from which both an N-terminal and a C-terminal fragment can be measured, abbreviated PINP and P1CP. Bone-specific alkaline phosphatase, BALP, is also released.(22)
Of these markers the urinary NTx and the serum osteocalcin are the most available and specific.
Currently there are no definitive guidelines on the use of bone markers because of the tremendous variability in the values in a single patient and among patients in a single group. Values may vary with age, sex, season of the year and time of day the specimen is taken. Young children and adolescents normally have very high levels. Secretion of markers tends to peak in the early morning hours. Food intake and kidney function will affect levels. When you take a marker, it must be compared to standardized tables of the age and sex of the patient. Taking two baseline specimens under identical conditions improves accuracy. When comparing a subsequent specimen with the baseline, for a change in a marker to achieve significance, it must be over and above the "least significant change" (LSC) due to the variations in assay and within the individual. The LSC for most markers is 30-40%.(22),(23)
Nevertheless, there are several reasons to use a bone metabolism marker in a given patient. The marker will show the effect of treatment much faster than bone density measurements, which require two years of an intervention to register a significant change. A second reason is to determine whether the patient has high or low bone turnover. High levels of both resorption and formation markers, as compared to standards, indicate high bone turnover.
Improving Bone Strength and Preventing Fragility Fractures
There are three areas you must consider when treating a patient with osteoporosis: dietary intake, physical status and activity, and pharmacotherapy.
Diet: Intake of building blocks and deleterious substances.
Deficits in calcium intake: Adequate calcium and vitamin D are required for success in any kind of pharmacotherapy whether the mechanism for building new bone is by increasing formation or stopping resorption. No matter what, the building blocks have to be available. Postmenopausal women should ingest 1200-1500 mg of elemental calcium daily; other groups, 1000 mg. The main sources of calcium in the western diet are dairy products. Patients with lactose intolerance or with malabsorption, notably subclinical celiac disease, may be particularly susceptible to low calcium absorption.(11) Look for and correct these underlying conditions to assure that calcium intake and absorption are adequate. Patients unable to ingest the recommended calcium amount in the daily diet can take supplements.(10)
Vitamin D intake: Adequate vitamin D is required for optimal calcium absorption. Vitamin D also improves muscle function, probably through its effect on muscle protein synthesis. Higher vitamin D intake has been significantly associated with a reduction in the number of falls among older people.(12) The RDA of 400 IU of Vitamin D may not be adequate for the elderly, so supplementation to 800 IU is recommended for that age group.
Alcohol and caffeine: High intakes of each of these substances are risk factors for OP, inquire and recommend moderation in both.
Smoking: Another risk factor. For innumerable reasons, in addition to OP, work with these patients to help them stop smoking.
Exercise and Activity: Bone growth is stimulated by physical stress. Life-long weight-bearing exercise is the best insurance for building peak bone mass and preventing critical age-related decline. Activities in the upright posture -- walking, running and jumping -- are most effective. An exercise prescription to initiate and increase regular exercise may motivate patients to change their attitude and activity.
Falls: Many factors contribute to falls. In addition to sedentary lifestyle and poor conditioning, a patient may have problems with balance and vision. Medications that decrease alertness and coordination, or lead to postural hypotension and hazards around the home can all cause falls. Advancing age increases the risk that a fall will result in a fracture, not only because of increased bone fragility, but as the result of decreased muscle mass and coordination. A targeted evaluation of a fall, and especially a series of falls, followed by specific interventions can reduce fall frequency. Evaluate the patient and treat medical causes (e.g., cardiac, neurological disease, excess medication). If home hazards are etiologic, arrange to identify and correct them. Prescribe exercises that build muscle strength and improve balance and coordination. Specific interventions can all decrease the risk of falling and lessen the consequences.(8)
Pharmacotherapy (In Order of Appearance)
Estrogen
Estrogen stimulates bone formation in endosteal sites and inhibits bone resorption. The Women's Health Initiative showed decreased hip and vertebral fractures in patients taking estrogen.(13) Estrogen increases bone density during the time of treatment but as soon as estrogen is stopped bone density starts decreasing.(14) Because the Women's Health Initiative did not show the protective effect against heart disease that we had assumed was operative and because of the increased incidence of breast cancer, estrogen is no longer recommended for primary prevention of osteoporosis. It may be used with careful monitoring for OP treatment in patients who cannot be helped by other agents.
Calcitonin (Miacalcin Nasal Spray or Injection)
This 32-amino acid peptide derived from the parathyroid glands modulates serum calcium levels by inhibiting resorption, primarily at vertebral sites. This action is mild compared with other antiresorptive agents. No study has demonstrated a significant decrease in fracture risk. Calcitonin has the interesting effect of decreasing the pain of recent vertebral fractures.(17)
Calcitonin must be given parenterally or transmucosally. The preparation mostly used for osteoporosis is Miacalcin Nasal Spray in a dose of 200 micrograms daily, delivered by one activation of the nasal spray. The main side effect is nasal irritation. Allergic reactions can occur but are uncommon.
The Selective Estrogen-Receptor Modulators (SERMs)
Estrogen-like compounds that have differing effects on the sites of estrogen action have been developed. Tamoxifen is antagonist on breast tissue and decreases recurrence and secondary primaries in postmenopausal breast cancer patients. Tamoxifen does have weak agonist effects on bone and causes an increase in bone density. Raloxifene (Evista) was specifically developed to treat osteoporosis and has a stronger agonist effect on bone, midway between estrogen and tamoxifen. Raloxifene decreases vertebral fracture incidence but not hip fractures. One proposed mechanism is that the antiresorptive effect of raloxifene is sufficient to stop osteoclasts from perforating the trabeculae, preserving the cancellous bone architecture, but that a stronger antiresorptive drug or bone-forming drug is required to increase cortical bone. Raloxifene is antagonist on the uterus, so avoids the problems of tamoxifen, and also decreases the incidence of estrogen-positive breast cancer.(15) Raloxifene may increase hot flashes and venous thrombosis. Other SERMs are currently being developed, so stay tuned.(10)
The Bisphosphonates: Alendronate, Risedronate and Ibandronate
These compounds bind to the active resorption sites in bone and inhibit the action of osteoclasts. Both alendronate (Fosamax) and risedronate (Actonel) have been shown to increase bone density and reduce fracture rate at both vertebral and nonvertebral sites compared with placebo. Both are oral preparations and can be taken for prevention or treatment of OP. Although initial studies were on daily dosage, further research has now shown that both drugs can be taken weekly. This is fortunate because bisphosphonates can cause GI irritation, especially in the esophagus. They can cause esophagitis or esophageal ulceration, particularly if the pill gets retained in the esophagus or gastric secretions containing the drug get washed back up into the esophagus. Patients should take their medication on an empty stomach, first thing in the morning, followed by a full glass of water and remain NPO and in an upright position for at least one-half hour. Bone and muscle pain and ocular inflammation have also been reported but are infrequent.(16)
The dosages of alendronate are 5 mg/day or 35 mg/week for prevention and 10 mg/day or 70 mg/week for treatment of OP. For risedronate the doses are 5 mg/day and 35 mg/week for both indications.
The new kid on the block is ibandronate (Boniva), approved in 2005 for a daily dose of 2.5 micrograms or a monthly dose of 150 micrograms. It has the same GI side effects as the other bisphosphonates but monthly exposure will decrease the risk and frequency of side effects. Rare cases of ocular inflammation have been observed but not the severe bone, joint and muscle pain.(16)
Zoledronate, an intravenous bisphosphonate used for hypercalcemia and malignancies, is now being tested as a once-a-year treatment in postmenopausal women.(10)
Parathyroid Hormone (PTH)/Teriparatide (Forteo)
PTH is an 84-amino acid peptide and teriparatide is a fragment of the first 34 amino acids of PTH, which is the biologically active segment. PTH can either break down or build up bone, depending on the type of exposure. Continuous infusion of PTH or chronic exposure to elevated levels of PTH, as in hyperparathyroidism, increases bone catabolism. On the other hand, intermittent exposure, as in a daily dose, increases bone density and strength. The mechanism is increased bone formation both at periosteal and endosteal sites, so that both cortical and trabecular bone are strengthened. Higher bone mineral density and decreases in fracture rate have been demonstrated in both types of bone. Bone mineral density increases more in trabecular bone than cortical bone but cortical bone strength is enhanced because periosteal apposition occurs, increasing the diameter and hence the resistance to bending.
Teriparatide must be administered parenterally. The dose is 20 micrograms subcutaneously every day. The FDA has put a limit of two years on treatment because osteosarcoma was seen in growing rats given high doses of the drug for two years. No cases have been seen in humans given the drug or in humans with hyperparathyroidism.
Patients should be cautioned about orthostatic hypotension, which has been seen in some patients after the first few doses of the drug. Mild hypercalcemia and hypercalciuria have occurred. Patients must be taking adequate calcium and vitamin D when on teriparatide because of its strong action on bone formation.Cost is an issue with teriparatide -- more than $500/month compared with $60-$70/month for raloxifene and the bisphosphonates.(19)
Some Observations About the Pharmacotherapies
I think you can see from the above discussion that in the last 10 years we see osteoporosis pharmacotherapy transformed and improved. We now have three bisphosphonates, one SERM and parathyroid hormone. We have learned a great deal about how all the OP drugs work, separately and together, and we need to change our thinking about some drugs we used before. Let me give you a quick run-down of what you should be thinking about.
Estrogen can no longer be considered a first-line drug for either prevention or treatment of postmenopausal osteoporosis (PMOP). While a woman is using estrogen in the immediate menopausal period, she will not have that sharp drop-off of BMD but we now know that when estrogen is discontinued, the drop in BMD will occur. Any woman over 65 and any woman younger than 65 with OP risk factors who is discontinuing estrogen should have a BMD test.(3) If osteoporosis is present, alendronate would be a good choice because it has been shown to sustain bone density after the withdrawal of estrogen. It stops that increase in resorption that occurs with estrogen withdrawal.(14)
Calcitonin should also be considered a second-line drug. The bisphosphonates, SERMs and PTH are all more effective and are backed up by strong evidence. Calcitonin's analgesic effect on painful vertebral fractures can be a helpful adjunct in some patients.
Alendronate (Fosamax) has been available in the U.S. for nearly 10 years, and a study reporting on 10 years of treatment with the drug showed increasing bone mineral density in vertebral sites and stable BMD at other sites. During the five years after treatment was discontinued, BMD decreased slowly. Because the bisphosphonates are incorporated into the bones, there has been a concern that over time the bones might become more brittle. This effect has not been seen in these recent observations, which is somewhat reassuring.(19)
How do the drugs compare? PTH causes the most gains -- 8% per year in the spine compared with 3-4% per year by alendronate. Raloxifene is in about the same range as the bisphosphonates.
Because PTH increases bone formation and bisphosphonates arrest resorption, will using both together result in greater gains in bone strength? It turns out that the effects are not additive. BMD gain with a combination of PTH and alendronate was midway between gains by each drug separately, PTH causing the greatest gains.(20)
On the other hand, using the drugs sequentially is advantageous. Alendronate sustains bone density after the withdrawal of estrogen or parathyroid hormone treatment.(14),(21)
Using Bone Mineral Density and Bone Markers to Aid Therapy
Bone density changes, as measured by DXA, are only truly significant after two years of treatment. Markers, however, can show a significant change in 3-6 months. This reassurance can be particularly useful in severely osteoporotic patients who are at high risk for fracture. In such patients, it is better to find out sooner, rather than later, that the patient is either not responding to or not complying with the treatment. When the bisphosphonates first came out in daily dosage form, noncompliance was frequent because of the inconvenience of dosage and high risk of GI side effects. Many prescriptions were not filled or refilled.(22)
Another use of markers is to determine whether a patient with osteoporosis has high bone turnover or low turnover. Someone with high turnover (high values of both resorption and formation markers) is a good candidate for anti-resorptive medications. A patient with low turnover would not be expected to improve bone formation by slowing resorption, since resorption is low to begin with and resorption and formation are coupled. That patient would be a candidate for teriparatide, which stimulates formation.
Summary
Osteoporosis is a seriously underdiagnosed and undertreated condition. We have preventive guidelines, in particular for postmenopausal women, and we need to follow those guidelines and treat detected cases. Just as importantly, we should diligently try to detect current cases and test high-risk patients, male or female, so that we can intervene in a timely way. This Cyberounds® provides you with practical information to sharpen your clinical skills and to improve your knowledge about testing procedures so that you can approach the diagnosis and management confidently. The Cyberounds® also gives you a run-down of the current treatments and follow up procedures.