Course Authors
Peter Barland, M.D.
Dr. Barland reports no commercial conflicts of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss the potential roles of B cells in rheumatoid arthritis
Describe the efficacy of rituximab and abatacept in rheumatoid arthritis
Describe the role of costimulation in T cell activation.
Dr. Barland will discuss the unapproved use of rituximab, abatacept and belimumab in the treatment of rheumatoid arthritis.
In an earlier Cyberounds, we discussed new drugs for rheumatoid arthritis (RA) that inhibited the proinflammatory cytokine TNF-α. Since then these anti-TNF-α drugs (etanercept, infliximab and adalumimab) have proven to be remarkably and enduringly efficacious when used alone and in conjunction with other disease modifying agents such as methotrexate and leflunomide (Figure 1).
Figure 1. Percentage of Patients Achieving ACR Goals: TEMPO Trial.*
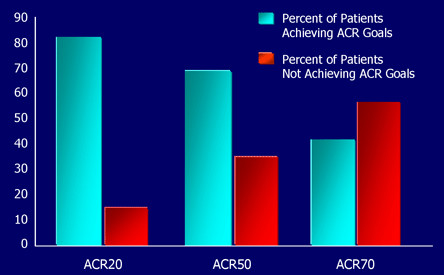
* Etanercept plus MTX arm.
Klareskog L et al. Lancet. 2004; 363:675-681
Results from the TEMPO trial show the ACR responses to etanercept and MTX therapy in RA after 12 months.
Many patients exhibit a dramatic improvement in their symptoms and approximately one-third appears to achieve a sustained remission of their disease.
Another benefit of these agents is their ability to reduce the rate of structural joint damage, which often progresses in RA despite an absence of clinically overt synovitis.
For Some, Anti-TNF-α Is Not Enough
However, a substantial number of patients do not respond or respond incompletely to anti-TNF-α therapy, while others develop adverse reactions and must cease treatment. In one retrospective study from a "real-world" practice, one-third of patients started on infliximab had discontinued the drug at 24 months.(1) The most common reason for discontinuation was a loss of efficacy (Figure 2).
Figure 2. Deficits of RA Therapy.
|
ACR, American Collge of Rheumatology.
* Feltelius N et al. Ann Rheum Dis. 2005; 64:246-252
Figure 2 lists justifications for additional therapeutic agents in RA.
New Drugs in Trial
Thus, there is clearly a need for additional agents to treat RA. Two biologic drugs, rituximab and abatacept, have recently been tested in Phase II trials and appear to provide effective alternatives to existing anti-TNF-α drugs in the treatment of RA.
Rituximab (RTX)
Rituximab (RTX) is a monoclonal antibody directed against CD20 -- membrane protein found specifically on B lymphocytes. CD20 is found on B cells from the time these cells acquire antigenic specificity until the time that they begin to mature into immunoglobulin secreting plasma cells (Figures 3, 4).
Figure 3. B Cell Membrane Markers.
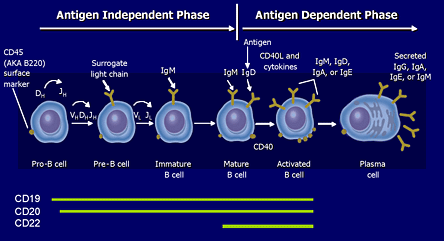
Adapted from Sell S et al. Immunology, Immunopathology, and Immunity. 6th ed. Washington, D.C.: ASM Press; 2001. Roitt I et al. Immunology. 6th ed. Philadelphia, PA: Mosby; 2001. Tedder TF et al. J Immunol. 1985; 135:973-979.
CD20 (and CD19) are membrane markers found on the surface of B cells during a defined portion of their maturation.
Figure 4. CD20.
|
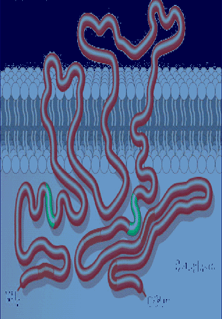 |
Johnson P et al. Semin Oncol. 2003; 30:3-8; Golay J et al. Blood. 2000; 95:3900-3908.
Immature pre-B cells residing in the bone marrow and mature plasma cells do not exhibit this membrane marker. RTX is currently approved for the treatment of relapsing non-Hodgkin's B cell lymphoma.
RTX Trials
Two large, prospective, double-blind, placebo-controlled trials have reported that RTX is effective in the treatment of RA patients with active disease despite DMARD treatment.(2),(3) (In both studies, patients who were currently on or had failed prior anti-TNF-α therapy were excluded.)
In the first trial, 161 patients were randomized into four groups. One group was continued on oral methotrexate (MTX) and, in addition, received placebo infusions. The second group received MTX plus two infusions of 1000 mg of RTX. A third group received two infusions of RTX, two infusions of cyclophosphamide three days after the RTX and placebo instead of MTX. The fourth group received RTX alone and placebos instead of MTX and cyclophosphamide. All patients received infusions of methylprednisolone after the RTX or placebo infusions and oral steroids for the first two weeks of the study (Figure 5, 6).
Figure 5. Rituximab Treatment Protocol.
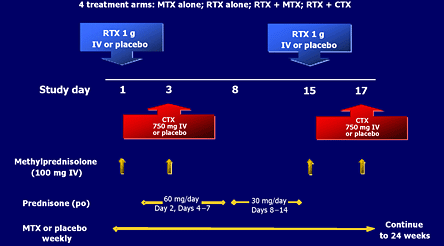
Edwards JCW, Szczepariski L, Szechiriski J, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. NEJM 2004; 350:2572-2581.
Figure 5 outlines the treatment protocol in pivotal rituximab trial.
Figure 6. Rituximab Trial: End-Points.
| 24 Weeks (Primary; LOCF)* | 48 Weeks (Exploratory; NRI) | 104 Weeks (CA) | |
| Efficacy | Primary
|
Durability of response All withdrawals considered non responders for ACR and EULAR outcomes | All withdrawals considered non responders for ACR and EULAR outcomes |
| Pharmacodynamic |
|
||
| Safety |
|
||
* Primary analyses done on last observation carried forward (LOC F) population NRI, nonresponders imputed
CA: completer's analysis
Figure 6 shows evaluation parameters for the same study.
The primary end point was the ACR50 response at 24-weeks; other responses were acquired as secondary end points and many of the patients were followed for 48 weeks. As shown in Figure 7, 40% of the patients in the combined RTX groups achieved an ACR50 response compared with 13% in the group that received MTX alone -- a statistically significant difference.
Figure 7. Rituximab Trial Results.
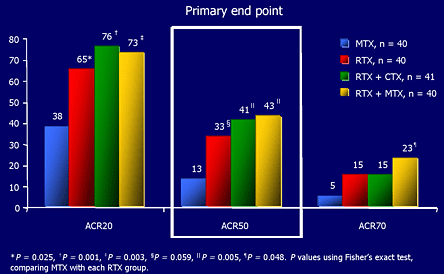
Edwards JCW et al. N Engl J Med. 2004; 350: 2572-2581.
Figure 7 shows primary results of the pivotal rituximab study.
Even more impressive was the observation that 20% of he patients who received RTX and MTX reached an ACR70 response compared with 5% of the patients on MTX alone. Many of these patients were considered to be in remission according to a DAS scoring system. [DAS, the Disease Activity Score, is a standardized and validated scoring system for measuring the clinical activity of RA that is used widely, especially in Europe.] Even at 48 weeks, without any further RTX treatment, a significant benefit persisted in the RTX treated patients (Figure 8).
Figure 8. Rituximab's Durability of Response.
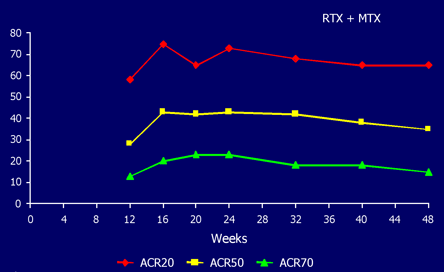
Preliminary data.
Emery P et al. Arthritis Rheum. 2003; 48:S439.
Figure 8 shows the persistence of responses to rituximab at 48 weeks after initial two infusions.
In all of the groups receiving RTX, there was a greater than 90% decrease in the number of circulating B cells that persisted for 24 weeks. By 48 weeks, there was a gradual and incomplete recovery of the peripheral B cells (Figure 9).
Figure 9. Rituximab and B Cell Activity.
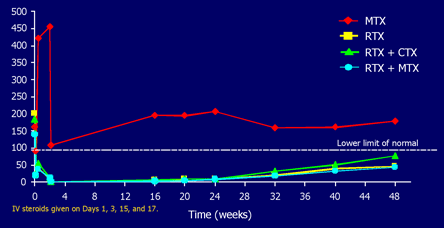
Edwards JCW et al. N Engl J Med. 2004; 350:2572-2581; Emery P et al. Arhtritis Rheum. 2003; 48:S438 (Preliminary data).
Figure 9 shows the depletion in circulation B cells after rituximab infusions.
Despite the observed virtual absence of B cells in these patients, there was no significant fall in the titer of preexisting anti-tetanus antibodies, though a transient fall in rheumatoid factor was noted. [N.B. Falling anti-tetanus antibody titers could indicate that patients might be susceptible to diseases to which they had been previously immunized.] Except for rapidly reversible infusions reactions after the RTX that could usually be prevented by slowing the rate of the infusion, there were few significant adverse reactions and no reported differences between the treatment groups (Figures 10, 11).
Figure 10. Adverse Effects in Rituximab Trial.
| MTX (n = 40) | RTX (n = 40) | RTX + CTX (n = 41) | RTX + CTX (n = 40) | |
| All events* 0 -- 48 weeks | 145 (85%) | 169 (88%) | 161 (85%) | 138 (85%) |
| Most frequently reported AEs (> 5%) | ||||
| RA exacerbation | 55% | 40% | 37% | 18% |
| Hypotension+ | 18% | 30% | 29% | 18% |
| Hypertension+ | 15% | 18% | 7% | 25% |
| Nasopharyngitis | 15% | 10% | 7% | 15% |
| Arthralgia | 8% | 8% | 5% | 13% |
| Back pain | 8% | 13% | 7% | 3% |
| Hyperglycemia | 15% | 5% | 7% | 8% |
| Cough | 15% | 5% | 8% | |
| Flushing | 8% | 13% | 5% | 3% |
| Headache | 5% | 5% | 7% | 8% |
* Number of events reported by percentage of patients.
+ Hypotension/hypertension defined as >30 mmHg change.
Edwards JCW et al. N Engl J Med. 2004; 350: 2572-2581.
Figure 10 lists the adverse reactions seen in different treatment groups.
Figure 11. Detailed SAEs in Rituximab Therapy.
| MTX (n = 40) | RTX (n = 40) | RTX + CTX (n = 41) | RTX + CTX (n = 40) | |
| Corneal abscess | 1 | |||
| Pneumonia (pseduomonal) | 1 | |||
| Bronchopneumonia* | 1 | |||
| Septic arthritis | 1 | 1 | ||
| Septicemia | 1 | |||
| Tendon rupture | 2 | |||
| Lumbar vertebral fracture | 1 | |||
| Anemia | 1 | |||
| Renal impairment | 2 | |||
| Pregnancy/abortion | 1 | |||
| Thrombosis | 1 | |||
| Pericarditis | 1 | |||
| Gastroenteritis (viral) | 1 | |||
| Goiter | 1 | |||
| Arytenoiditis | 1 | |||
| Migration of renal stent | 1 | |||
| Total | 3 | 4 | 8 | 4 |
*Preliminary data.
1-24 weeks; 25-48 weeks.
* Patient death
Szczepaski L et al. 67th Annual Meeting of the American College of Rheumatology, October 23-26, 2003; Orlando, Florida.
Figure 11 details all serious adverse reactions in the different treatment groups.
Another prospective, placebo-controlled, 24-week study compared two doses of RTX (500 mg/infusion and 1000 mg/infusion) with placebo in active RA patients continued on MTX. The patients were also randomized into steroid and no steroid treatment groups (Figure 12).
Figure 12. Rituximab in DANCER Trial.
| Rituximab | ||||
| Placebo | 2 x 500 mg | 2 x 1000 mg | ||
| Glucocorticoids | Placebo | 41 | 41 | 41 |
| IV premedication | 39 | 41 | 41 | |
| IV premedication + oral | 42 | 41 | 40 | |
RF+ITT population (n=367)
RF- (n=84)
Figure 12 outlines the protocol for the second rituximab study in RA which evaluated different doses of rituximab and effect of corticosteroids.
The results are comparable to those of the previous study, although the group receiving 500 mg of RTX per infusion had lower ACR70 responses (Figure 13).
Figure 13. Rituximab: Results from DANCER.
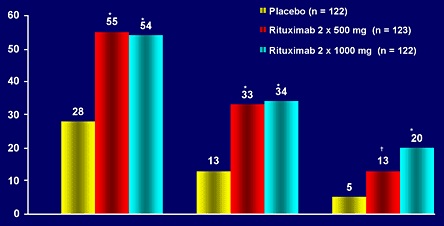
*P<0.0001 rituximab vs. placebo (logistic regression: contrast test in RF positive patients [ITT population]); +P=0.020.
Withdrawals or patients with insufficient data to calculate an ACR score were classed as nonresponders.
Emery P et al. Annual Eurpoean Congress of Rheumatology (EULAR). June 8-11, 2005; Vienna, Austria. Abstract OP0008. Ann Rheum Dis. 2005; 64 (suppl 3):58.
Figure 13 notes the primary results of the rituximab dosing study (DANCER).
The use of steroids had no effect on the results at 24 weeks, although they did appear to decrease the frequency and intensity of the infusion reactions. The number of serious adverse events was comparable in all groups. A small number of patients were retreated with a second course of RTX after 48 weeks when disease activity returned. Most of these patients noted a significant benefit from the second course.
RTX appears to be a safe and effective agent for the treatment of RA. Currently, there is no published information on the effect of RTX on structural joint damage, on the efficacy of RTX in patients who have failed anti-TNF-α therapy and on the efficacy of RTX for more than two years.
Beyond the T Cell
For many years, immunologists believed that RA was primarily mediated by T cells. However, the results observed with RTX indicate that B cells play an important role in the pathogenesis of the disease. Experimental evidence further supporting a vital role of B cells in RA comes from studies in which rheumatoid synovial tissue is explanted into SCID mice that lack both T and B cells. When these mice are treated with RTX, there is marked decrease in inflammatory cytokines produced by the explanted tissue compared with control mice treated with normal immunoglobulin (Figures 14-16).(4),(5)
Figure 14. Rheumatoid Synovitis.
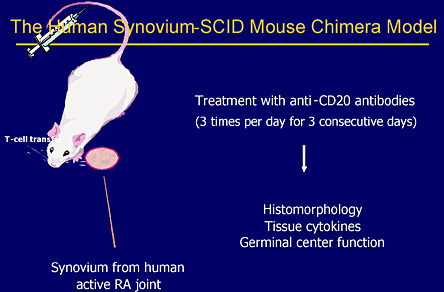
Figure 14 illustrates the experimental protocol used to show role of B cell in rheumatoid synovitis.
Figure 15. Rituximab's Effect on Cytokines.
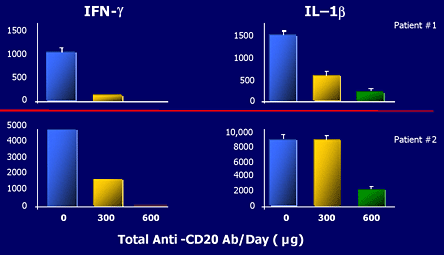
Human synovium -SCID chimera grafts retrieved 6 days after RTX injections.
Takemura S et al. J Immunol. 2001; 167:4710-4718.
Figure 15 demonstrates the effect of rituximab on proinflammatory cytokines (IFN gamma and IL1 beta) in rheumatoid synovial explants in SCID mice.
Figure 16. Rituximab and Histology of RA.
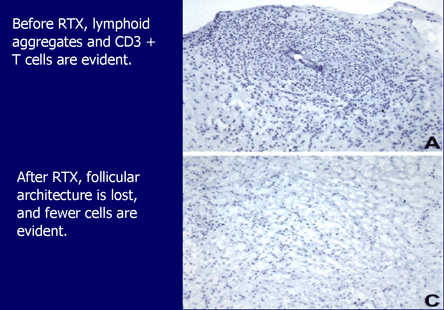
Following B-cell depletion, fewer cells are evident.
Takemura S et al. J Immunol. 2001; 167:4710-4718.
Figure 16 Shows the histological changes in the rheumatoid explants after treatment with rituximab.
Researchers have hypothesized several ways that B cells may act to initiate or perpetuate the immunoinflammatory response seen in RA. They may present antigen to T cells, secrete pathogenic antibodies such as anti-CCP antibodies or produce proinflammatory cytokines (Figure 17). Which of these B cell functions are operative in RA is not known.
Figure 17. B Cells and Immunoresponse.
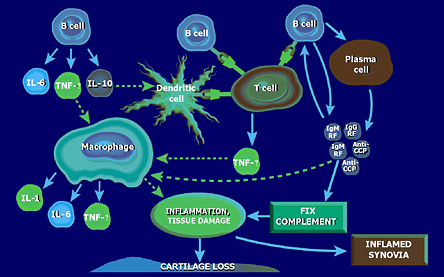
Edwards JC et al. Immunology. 1999; 97:188-196; Takemura S et al. J Immunol. 2001; 167:4710-4718; Albers B et al. Molecular Biology of the Cell. 3rd ed. New York, NY: Garland Publishing; 1994; Metlay JP et al. Adv Immunol. 1989; 47:45-116; Dorner T et al. Curr Opin Rheumatol. 2003; 15:246-252; Pistoia V et al. Stem Cells. 1995; 13:487-500.
Depiction of the potential roles of B cells in the immunoinflammatory reaction seen in RA.
Belimumab, B Cell Growth Factor
B cells also require a growth factor known as BLyS (B lymphocyte stimulator) for maximal proliferation and maturation. Preliminary data from trials using a monoclonal antibody to BLyS, belimumab, indicate that this agent also has therapeutic efficacy in RA.(6)
CTL4A and Abatacept
The antigenic activation of T cells requires recognition of antigen in the context of a Class II HLA molecule on an antigen-presenting cell by the specific T cell receptor. Activation also requires a second signal through interaction between a ligand on the antigen-presenting cell and a receptor molecule on the T cell.
One of the most important of these costimulator pairings is between CD80 and CD86 on the antigen-presenting cell and CD28 on the T cell. This interaction is modulated by the production of another T cell membrane protein, CTLA4. CTLA4 binds more avidly to CD80 and CD86 than CD28 and inhibits further T cell activation.
Abatacept is a recombinant fusion protein comprising the extracellular domain of human CTLA4 and a fragment of the Fc chain of human IgG1. Abatacept, like CTLA4, competes with CD28 for CD80 and CD86 binding and thereby selectively modulates T cell activation.
The response to abatacept has been reported from two phase II, prospective, randomized studies in RA. In one trial, 329 TNF-α naive patients with active RA despite MTX were treated with continued MTX and either abatacept by intravenous infusions or placebo and followed for 12 months.(7),(8) The abatacept patients had significantly greater ACR20 (63% vs. 36%, P < 0.001), ACR50 (42% vs. 20%, P < 0.001) and ACR70 (21% vs. 8%, P < 0.003) responses. No serious adverse reactions or serious/opportunistic infections were encountered.
In a second study, 391 patients with active RA, despite current or previous treatment with anti-TNF-α, were randomized to receive abatacept or placebo. Both groups continued on their previous DMARD therapy but all TNF-α inhibitor therapy was stopped a minimum of 30 days before commencing the trial drug. At six months, the abatacept patients again achieved significantly greater ACR20 (50% vs. 20%, P<0.001), ACR50 (20% vs. 4%, P<0.001) and ACR70 (10% vs. 1.5%, P=0.003). The patients receiving abatacept also reported improvement in physical function. The incidence of serious adverse reactions was similar in the two groups and no unusual or opportunistic infections were observed.
It should be noted that there have been anecdotal reports of serious and unusual infections seen in patients treated with abatacept, so if this drug is approved it will probably be with a caution concerning the possibility of infection and a prohibition against use with other immunosuppressive biologics.
Neither trial reported structural damage assessment. Based on these well-controlled studies, it appears that abatacept is a potentially useful new agent in the treatment of RA, including patients that have failed anti-TNF-α therapy.
Hopefully, one or both of these new drugs (only rituximab and abatacept have filed with the FDA) will be approved for the treatment in the next year and will provide patients with additional options. These agents, like the anti-TNF-α drugs, are expensive and it will be interesting to see how the third party payers position their reimbursement policies. Until more data is available it is not recommended that more than one biologic agent be used at the same time.