Course Authors
Russell D. Romeo, Ph.D., and Bruce S. McEwen, Ph.D.
Dr. Romeo is a Fellow, Laboratory of Neuroendocrinology, The Rockefeller University, New York.
Drs. Romeo and McEwen report no commercial conflict of interest.
This activity is made possible by an unrestricted educational grant from Forest Laboratories.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Identify the two major classes of glutamate receptor
Describe the subunits and subtypes of these glutamate receptors
Discuss the contribution of these receptors to synaptic transmission and plasticity.
Glutamate receptors are responsible for most of the excitatory neurotransmission in the mammalian brain and spinal cord. There are two major types of glutamate receptors, the ionotropic and metabotropic receptors. The ionotropic receptors function as glutamate-gated ion channels, while the metabotropic class of glutamate receptors signal through intracellular G-proteins that bind to guanosine diphosphate (GDP) or guanosine triphosphate (GTP), which in turn, modify the activity of other receptors and ion channels.
The expression of the glutamate receptors changes depending on the anatomical location and developmental age of the organism. These receptors contribute to such diverse phenomena as learning and memory, drug sensitivity and neuronal degenerative diseases. The purpose of this Cyberounds® is to provide a brief review on the general structure, function and anatomical distribution of ionotropic and metabotropic glutamate receptors.
Ionotropic Glutamate Receptors
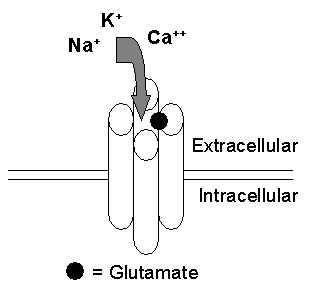
There are three types of ionotropic glutamate receptors: the closely related alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptors, kainate receptors and the N-methyl-D-aspartic acid (NMDA) receptors. These receptors mediate rapid excitatory neurotransmission in such brain regions as the hippocampus and cortex. They are classified as ionotropic because they are ligand-gated channels (i.e., the channel gate is opened or closed by its ligand glutamate) that allow ions to travel into the neuron (Figure 1A).
Figure 1A. A Schematic of a Glutamate-Gated Ionotropic Glutamate Receptor.
Although each ionotropic glutamate receptor has its own particular structure and function (discussed below), they do share a number of common characteristics. For instance, each of these receptors is composed of multiple subunits (four or five) that have four membrane domains (Figure 1B), three of which traverse the plasma membrane (e.g., M1, M3 and M4) and one re-entrant loop (e.g., M2).(1)
Figure 1B. Schematic of the Three Transmembrane Domains (M1, M3 and M4) and the Single Re-Entrant Loop (M2) of an Ionotropic Glutamate Receptor Subunit.
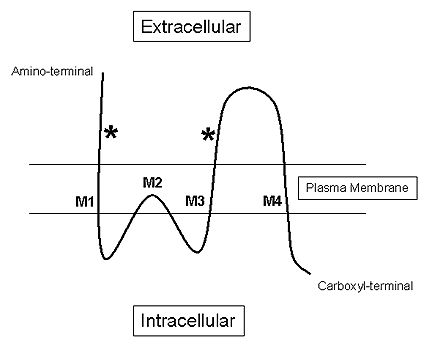
The "*" indicates glutamate binding sites.
Furthermore, the extracellular amino-terminal domain of these receptors contains the glutamate binding site, while the intracellular carboxyl terminal of these receptors mediates various functions such as receptor phosphorylation, signal transduction and interactions with intracellular scaffolding and anchoring proteins. Of the three ionotropic glutamate receptors, the AMPA and NMDA receptors are the most well studied, but recent advances in more selective pharmacological ligands for kainate receptors will undoubtedly begin to elucidate a more exact pharmacology of this ionotropic glutamate receptor subtype.(2)
AMPA Receptors
The AMPA receptors are composed of four subunits: GluR1, GluR2, GluR3 and GluR4 (also known as GluRA-D). However, each subunit may not be represented in a single receptor such that an AMPA receptor may be comprised of two GluR2 and two GluR3 subunits, for instance. The AMPA receptors can allow Na+, K+ and Ca++ to pass. However, they primarily conduct Na+ and K+ ions. The conductance of the receptor will depend on which subunits compose the receptor. For example, AMPA receptors that contain the GluR2 subunit are impermeable to Ca++.(3)
Binding studies have shown that the AMPA receptors are highly enriched in the hippocampus and cerebral cortex.(4) However, lower levels of AMPA expression are also found in the diencephalon, midbrain and brainstem.(4) The expression levels of the subunits also change depending on anatomical location. Specifically, the GluR1, GluR2 and GluR3 subunits are highly expressed in the hippocampus, while the GluR4 subunit is abundantly expressed in the granule cells of the cerebellum.(5)
AMPA receptors can interact with intracellular proteins, as the cytoplasmic tails of the receptor subunits have one or more intracellular protein-protein interaction motifs known as PDZ domains. Receptors that contain GluR2 and GluR3 subunits can bind to and interact with the intracellular adaptor protein GRIP (glutamate receptor-interacting protein).
GRIP appears to be responsible for targeting and aggregating AMPA receptors at excitatory synapses.(6) Conversely, AMPA receptors with GluR1 subunits bind to and interact with SAP97 (synapse-associated protein 97), which may allow the AMPA receptors to interact with other glutamate receptors (e.g., NMDA receptors).(7) Other adaptor proteins have been identified [e.g., RIL (reversion-induced LIM gene), PICK1 (protein interacting with C-kinase)] that mediate AMPA receptor trafficking, linkage with the cytoskeleton and intracellular signaling.(8) Thus, in addition to the different ion permeabilities imposed by the subunit composition, the PDZ domains on their cytoplasmic tails can further affect the physiological properties of these receptors.
AMPA receptors cycle into and out of the plasma membrane through endo- and exocytosis and maintain a steady-state distribution on the surface of the membrane. However, upon stimulation, the AMPA receptors become more dynamic and can be either recruited to or away from the synapse. This active trafficking of the AMPA receptor is thought to mediate certain aspects of synaptic plasticity.(8) For example, AMPA receptors in the hippocampus have been shown to play a fundamental role in long-term potentiation (LTP) and long-term depression (LTD), putative electrophysiological correlates of learning and memory. During LTP, AMPA receptors are recruited to the surface of dendritic spines, which are postsynaptic specializations that receive excitatory transmission.(9)
On the other hand, AMPA receptors undergo endocytosis during LTD, which would make the receptor physically unavailable to bind glutamate on the cell surface leading to a reduction in the signaling capacity of the cell.(10),(11) Taken together, these studies highlight the importance of the physical location of the AMPA receptor and how activity-induced translocation of the receptor can have profound physiological consequences for the neuron.
NMDA Receptors
Five subunits make up NMDA receptors: NR1, NR2A, NR2B, NR2C and NR2D. Similar to the AMPA receptors, the function of the NMDA receptor is different depending upon its constituent subunits. However, all NMDA receptors posses an NR1 subunit, which is responsible for the channel activity of the receptor, while the particular NR2 subunits comprising the rest of the receptor play a more modulatory role.(5)
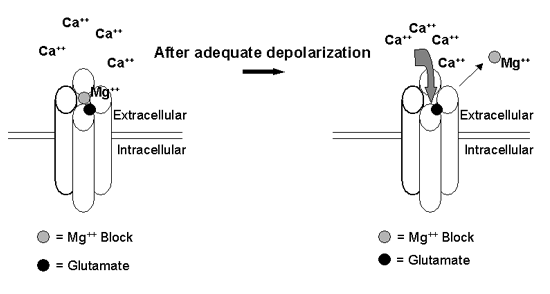
NMDA receptors are highly permeable to Ca++. Interestingly, NMDA receptors have a voltage-dependent Mg++ block that keeps the channel impermeable to Ca++ until the neuron reaches a critical depolarized state (Figure 2). This aspect makes the NMDA receptor unique among the ionotropic glutamate receptors.
AMPA receptors often work in tandem with NMDA receptors to provide the necessary depolarization to remove the Mg++ block. Indeed, AMPA and NMDA receptors are often co-localized at the same synapse.
It should be noted, however, that not all excitatory synapses posses both receptor subtypes. For instance, some synapses contain only NMDA receptors, which are inactive or "silent" at resting membrane potentials. These "silent" synapses can be activated upon stimulation by the rapid recruitment of the AMPA receptors to the membrane.(12) Conversely, it has been reported that a subtype of cerebellar synapses contain only AMPA receptors until sufficient glutamate concentrations are reached, at which time extrasynaptic NMDA receptors are recruited to the synapse.(13)
Mapping studies have shown that NMDA receptors are widely distributed throughout the brain.(5) Similar to the AMPA receptors, NMDA receptors are highly concentrated in the hippocampus and forebrain. Not surprisingly, the distribution of the NR1 subunit is similar to that of the receptor; however, the NR2 subunits show distinct regional specificity. For instance, NR2A and NR2B are highly expressed in the hippocampus and cerebral cortex, while the NR2C subunit is expressed primarily in the cerebellum.(14) NR2D is principally expressed in thalamus, midbrain and brainstem.(14) In addition to their different regional distributions, these subunits vary markedly in their temporal expression. NR2A and NR2C levels increase during postnatal development, while NR2B and NR2D levels peak at birth and decline thereafter.(14)
At the synapse, NMDA receptors are tightly associated with the postsynaptic density (PSD) via their PDZ domains. Recent evidence indicates additional intermediary adaptor proteins named GKAP (guanylate kinase-associated protein) and Shank (a novel family of postsynaptic density proteins that binds to the NMDA receptor/PSD-95/GKAP complex and cortactin) link the NMDA receptors to the PSD.(15),(16) These adaptor proteins are thought to cross-link PSD/NMDA receptor complexes to other glutamate receptors (i.e., metabotropic glutamate receptors via its adaptor protein Homer, see below) and the cytoskeleton.(15)
The linkage between the PSD and the NMDA receptor is also thought to stabilize the NMDA receptor at the postsynaptic membrane.(17) NMDA receptors can also be recruited or sequestered at synapses similar to that observed for AMPA receptors.(18) This NMDA receptor trafficking undoubtedly plays a role in both LTP and LTD.
As mentioned above, NMDA receptors are highly permeable to Ca++. Under physiological conditions this Ca++ influx allows for excitatory neurotransmission and synaptic plasticity. However, excessive Ca++ influx can lead to neuronal damage. Aberrant or overactive NMDA receptors can cause neuronal cell death associated with ischemia, seizure and neurodegenerative diseases (e.g., Parkinson's or Huntington's disease).(19)
Kainate Receptors
Kainate receptors are closely related to the AMPA receptors (e.g., kainate is a potent activator of AMPA receptors). These receptors are composed of 5 subunits: GluR5, GluR6, GluR7, KA-1 and KA-2. Similar to NMDA and non-GluR2-containing AMPA receptors, the kainate receptors are highly permeable to Ca++.
Kainate receptors are located throughout the brain and spinal cord, with a concentration in the hippocampus and cerebellum.(5) The KA-2 subunit is widely expressed in the central nervous system, while KA-1 appears to be primarily expressed in the hippocampus.(20) The GluR5, GluR6 and GluR7 subunits are most highly expressed in the cingulate cortex, cerebellum and the inner layers of the cerebral cortex, respectively.(20)
Like AMPA receptors, kainate receptors interact with similar intracellularadaptor proteins (e.g., PICK1 and GRIP) via their PDZ domains. These elements have been proposed to stabilize the kainate receptors at the synapse.(21)
Kainate receptors are located both pre- and post-synaptically. Postsynaptically, kainate receptors play a role in excitatory synaptic transmission, while presynaptically they modulate the release of excitatory and inhibitory neurotransmitters.(22) For instance, activated kainate receptors on the presynaptic element may inhibit or stimulate the release of glutamate or GABA from the presynaptic terminal. These presynaptic receptors also appear to mediate the induction of LTP in the hippocampus.(23) Thus, in addition to their AMPA receptor-like qualities, pre- and post-synaptic kainate receptors play neuromodulatory roles in excitatory and inhibitory neurotransmission and synaptic plasticity.
Metabotropic Glutamate Receptors
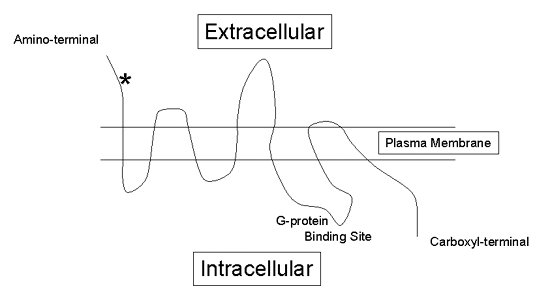
Metabotropic glutamate receptors are G-protein coupled receptors. There are eight different subtypes of metabotropic receptors (mGluR1-mGluR8) and all have seven transmembrane regions (Figure 3) similar to other G-protein coupled receptors (e.g., serotonin receptors).
The "*" indicates glutamate binding sites.
Interestingly, unlike other G-protein coupled receptors, the metabotropic glutamate receptors have rather large extracellular domains, the exact function of which is currently not well understood.(5)
The eight subtypes are divided into three groups based upon their agonist specificity and predominant intracellular signaling pathway. The group I metabotropic glutamate receptors are composed of the mGluR1 and mGluR5 subtypes. These receptors primarily signal through the phospholipase C (PLC) pathway that activates inositol-1,4,5-triphosphate (IP3) which, in turn, allows for the release of intracellular Ca++ stores.(24),(25),(26),(27)
It should also be noted that the mGluR1 receptor subtype signals through the cAMP pathway.(24) These group I receptors interact with Homer, an intercellular adaptor protein, that targets them to dendritic spines.(27) and links them to IP3 receptors, Ca++ and K+ channels and NMDA receptors.(28),(29),(30) The group II receptors include the mGluR2 and mGluR3 receptor subtypes, while the group III receptors are comprised of the mGluR4, mGluR6, mGluR7 and mGluR8 subtypes. Both the group II and III receptors inhibit adenyl cyclase signaling and decrease cAMP formation, which would ultamately result is less cellular activity and signaling.(5),(31),(32)
The metabotropic glutamate receptors have a wide distribution in the central nervous system. The mGluR1, mGluR3 and mGluR7 subtypes are highly expressed in the cerebral cortex and hippocampus.(31),(32), (33),(34) The mGluR2 and mGluR4 subtypes are enriched in the cerebellum(31),(35) and, in addition to the cerebellum, mGluR4 is highly expressed in the olfactory bulb, as is mGluR8.(31),(36) The mGluR5 subtype is primarily expressed in the striatum,(25) while the mGluR6 subtype is solely expressed in the retina.(37)
Similar to kainite receptors, several mGluR receptors subtypes have been localized at both the pre- and post-synaptic sites and have been shown to play a modulatory role in excitatory and inhibitory neurotransmission.(38) Presently, there appears to be little evidence that metabotropic glutamate receptors play a direct role in hippocampal LTP.(39) However, these receptors have been reported to mediate LTD in the cerebellum.(40)
In addition to their roles in synaptic plasticity, it appears that all three groups of metabotropic receptors may reduce excitotoxic neuronal degeneration (i.e., neuronal death induced by supraphysiological levels of glutamate).(41),(42) Furthermore, stimulation of group II and III receptors may protect against seizure activity, while overactive group I receptors may promote seizure activity.(43)
Conclusions
The ionotropic and metabotropic glutamate receptors are responsible for the majority of the excitatory neurotransmission in the central nervous system. Thus, it should not be surprising that there are a wide variety of these receptors types (and subtypes) with diverse anatomical distributions and temporal expressions. To add to this complexity, the different intracellular PDZ domains, with their respective adaptor proteins and the various signaling cascades induced by the metabotropic receptors, further contribute to the particular function and mechanism of action of each receptor type.
The continued elucidation of the structure and function of the ionotropic and metabotropic glutamate receptors is of paramount importance to understand how these receptors contribute to physiological and pathophysiological states.