Course Authors
Hany Awadalla, M.D., M.Sc., Abid R. Assali, M.D., and Richard W. Smalling, M.D., Ph.D.
Drs. Awadalla and Assali are Fellows, Cardiology Division, University of Texas Medical School and Hermann Hospital, Houston, Texas.
Drs. Awadalla and Assali report no commercial conflict of interest. Dr. Smalling has received grant/research support from Centocor/Boston Scientific within the last three years.
This presentation will include discussion of commercial products and services.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Describe, in general terms, the endovascular AAA repair technique
Identify those patients who may benefit from endovascular AAA repair
List the complications of endovascular AAA repair.
|
Case Presentation A 73-year-old white male with history of hypertension, hypercholesterolemia and tobacco smoking for 30 years was referred for possible abdominal aortic aneurysm (AAA) repair found on routine physical examination. Other medical history included stable angina pectoris. |
Physical Examination
P.E. revealed an obese white male in no apparent distress. Blood pressure was 145/86 mm Hg, pulse was 86 beats per minute, regular and well felt in all four extremities.
Neck: There was no jugular venous distension. Carotid pulses were 2+ bilaterally and a right -sided carotid bruit was appreciated.
Cardiac Examination: The PMI was not displaced. There was a soft S1 and S2 with a grade II/IV systolic murmur compatible with known mitral valve regurgitation.
Abdominal Examination: A soft slightly tender pulsatile mass was palpated in the left lower abdomen.
Extremities: The femoral and pedal pulses were 2+ without bruits or pedal edema.
Laboratory Investigations
The sodium was 135 mEq/ml, potassium 3.8 mEq/ml, blood urea nitrogen 13 mg/dl and serum creatinine 0.8 mg/dl. Hemoglobin was 16 gm/dl and hematocrit was 48 percent.
Medications
Patient was taking enteric coated acetyl salicylic acid 325 mg once a day orally, simvastatin 20 mg once a day orally, diltiazem sustained release preparation 300 mg orally once a day and lisinopril 20 mg once a day orally.
Imaging
- A CT angiogram of the abdomen and pelvis revealed an AAA that started 2 cm below the level of renal arteries, with intramural thrombus and mild calcification The measurements on the CT angiogram revealed a 2-cm proximal neck length and a 2.5-cm proximal neck diameter, a 7-cm length of the aneurysm body for a maximum of 5.3 cm aneurysm diameter, with a 0.5 cm lower neck length and a 2.5 cm lower neck diameter, 1.2 cm for the right iliac and 1.2 cm for the left iliac diameters. The length of the right and left iliac arteries was 5 centimeters bilaterally.
- Rotational angiography (Figure 1) pre-operatively showed a proximal aortic neck measuring 28 mm in length and 23.4 mm in diameter.

Figure 1.
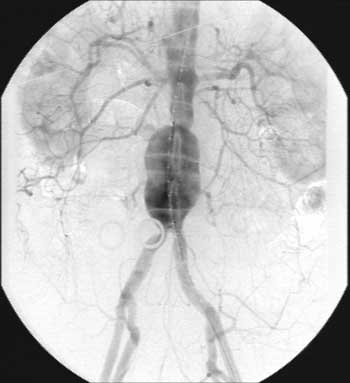

The superior mesenteric artery was normal without stenosis. The length of the distal aortic neck was 5 mm in length, while its diameter was 23.3 mm. The right common iliac artery was 52 mm in length and 13.7 mm in diameter, while the left common iliac artery was 59 mm in length and 10.6 mm in diameter. Total length measured from below the level of renal arteries to the level of common iliac bifurcation was 10.4 cm.

Table 1. Patient Imaging Data
| CT Angiogram | 3-D Rotational Angiogram | |
|---|---|---|
| Total length of aneurysm | 12 cm | 12 cm |
| Proximal neck diameter | 25 mm | 24 mm |
| Proximal neck length | 20 mm | 25 mm |
| Distal neck diameter | 25 mm | 23 mm |
| Distal neck length | 5 mm | 11.5 mm |
| Right common iliac length, Diameter | 50 mm, 12 mm | 52 mm, 13.7 mm |
| Left common iliac length, Diameter | 50 mm, 12 mm | 59 mm, 10.6 mm |

The patient was referred for AAA exclusion because of the size of aneurysm. The open surgical and endovascular repair options were explained to the patient. A decision was made to proceed with endovascular repair.
Procedure
The patient was taken to the angiography suite with operating room capabilities, placed in the supine position and administered general endotracheal anesthesia. The abdomen and groins were draped in the usual sterile fashion. Using the Seldinger's technique, a 12-Fr sheath was placed in the right common femoral artery.
Pre-repair coil embolization of a large inferior mesenteric artery was performed using a combination of Nester coils (Cook Inc, Bloomington, IN, USA) in sizes 4, 5 and 7 mm. (Sac branch vessel patency is associated with significantly higher early total and type 2 endoleak rates after stent-graft repair of AAAs; thus, patent sac branches play an important role in the pathogenesis of endoleaks.)(1)
A longitudinal incision was made in the left groin to expose the common femoral artery just below the inguinal ligament. Roummel tourniquets were placed proximally and distally. Heparin was given intravenously 100 units per kilogram body weight. A small transverse arteriotomy was made in the left common femoral artery and a pigtail catheter was advanced over a guide wire into the thoracic aorta. After aortography, the flexible wire was replaced with a super-stiff Lunderquist guide wire for axial support (Cook, Bloomington, IN).
The 25-Fr Ancure expandable sheath (consists of a sheath, a dual valve mechanism and a coaxial dilator system) was inserted in the left common femoral artery, with dilator in the iliac artery for two minutes over the Lunderquist guide wire. A Terumo glide wire (Terumo Corporation, Hatagaya, Shibuyaka, Tokyo) was then passed via the left sheath across the aortic bifurcation and retrieved through the right sheath using a 6-F internal mammary catheter.
The contralateral pull through wire of the device was then fed into the tip of the internal mammary catheter and retrieved from the left sheath. The Ancure delivery system (Guidant/EVT, Menlo Park, CA), housing the endo-vascular bifurcation graft measuring 26 mm X 14 mm and 15 mm, was then inserted over the super-stiff guide wire via the Ancure expandable sheath. Subsequently, the delivery system was passed to the desired level, with the proximal capsule above the aortic bifurcation.
The jacket lock in the delivery system was unlocked and retracted simultaneously with the contralateral pull wire uncovering the graft. Each limb of the graft was retracted simultaneously into the iliac arteries until the proximal attachment hooks were located just below the renal arteries. After reconfirming the position of the proximal endohooks attachment system, using the marker to delineate the level of renal arteries (and subtraction angiogarphy), the superior attachment system was deployed.
The hooks were secured in place by inflating the aortic balloon three times (each for approximately one minute). Subsequently, the right limb of the graft was attached to the Ancure contralateral torque catheter over the contralateral pull wire. The contralateral attachment system was deployed by cutting the fusion joint, holding the pull wire fixed and retracting the torque catheter. The hooks were secured in place by inflation of a standard PTA balloon (14 mm X 2 cm) up to 7 atmospheres for 20 seconds. The left limb of the graft was then positioned in the left common iliac artery and deployed above the iliac bifurcation. The ipsilateral attachment hooks were secured in place with inflation of another balloon (14 mm X 2 cm) up to 6 atmospheres for 15 seconds. Both balloons were inflated simultaneously up to 6 atmospheres for 10 seconds, incrementally up the graft limbs to the graft bifurcation. (Hence, both limbs of the graft were "ironed out.")
Following endovascular deployment, there were no significant systolic or mean pressure gradients [mean gradient was less than 2 mm Hg across the graft]. (A mean gradient >4 mm Hg between the two femoral arteries is associated with increased risk of post-procedure complications.)
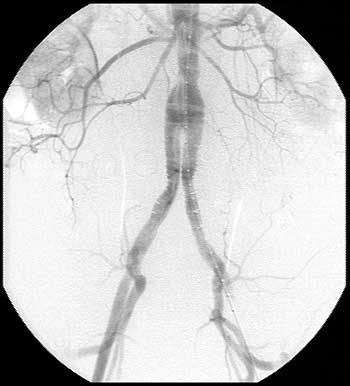
Final angiography (Figure 2) revealed no endovascular leak and good positioning of the endograft.

Figure 2.

The right common femoral artery puncture was repaired using a 10-F Perclose suture device (Abbott Laboratories Company, Redwood City, CA). The left common femoral arteriotomy was closed using continuous 5-0 prolene sutures. Hemostasis was then meticulously secured. The left groin incision was closed in layers using 2-0 PDS for the deep layers and the skin was closed with a running 4-0-subcuticular suture.
Patient was discharged 24 hours following the procedure, on the same pre-procedure medications, with excellent distal pulses and hemoglobin of 14.7 gm/dl.
A follow up CT scan 3 months post implant showed no endoleak.
Comments
The goal of treating AAA is to prevent rupture with acceptable rates of operative morbidity and mortality. The operative mortality rates for AAA were found to be 8.4% for elective surgical repairs and as high as 68% for emergency AAA repairs. Factors found to influence the outcome included AAA location, urgency of repair, patient sex and the size and geographic location of the hospital in which the procedure was performed.(2)
Indications for elective repair include non-symptomatic aneurysms more than 5 centimeters in diameter, all other symptomatic and ruptured aneurysms, provided coexisting medical conditions do not preclude the operation.(2)
The use of endovascular stent-graft prostheses for the treatment of infra-renal AAA is receiving increasing attention as an option that may avoid the significant morbidity and mortality associated with open surgical treatment. With the introduction of endovascular grafting as an example of a less invasive modality for treatment of AAA, some investigators postulated that it might lower the threshold of repair for AAA.(3)
Stent graft devices are available in various configurations -- tube grafts, aorto-unilateral and aortic bifurcated grafts. Currently, there are two FDA-approved devices (Ancure, Guidant/ EVT, Menlo Park, CA), and AneuRx, Medtronic, Santa Rosa, CA).
The available non-randomized trials comparing endovascular and surgical procedures vary in their conclusions. While some(4) concluded that there was no clear benefit from the less invasive procedures, as measured by duration of hospital stay or preoperative morbidity and mortality, others(5) report that endovascular treatment significantly reduces resource utilization and cost compared to trans-abdominal repair. Success rates of endovascular grafts may vary according to graft configuration (at 40 months the success rates for tube grafts is 50% and bifurcated grafts 80 %).(6)
Suitability of AAA for Endovascular Repair
Important anatomical considerations that were stressed since the introduction of endovascular treatment include:
- The proximal anchoring of the device as determined by the proximal neck dimensions. The proximal neck should at least be 15 mm in length and should not exceed 26 to 28 mm in diameter (depending on the device used). The angulation at the proximal neck should be less than 60 degrees.
- Distal neck (necessary for placement of tube grafts) length of at least 20 mm above the bifurcation of the iliac arteries is required for placement of tube grafts. Bifurcated stent grafts have been developed to overcome this limitation.(7),(8),(9)
- External iliac artery diameter, presence of circumferential calcification and tortuousity of the external iliac arteries are important factors for selecting patients and predicting procedural complications including perforation and conversion to open repair. The introducer sheaths of the stent graft systems range from 20-27 Fr. Since these sheaths are introduced through the arteriotomy, passing through the femoral and iliac arteries lumen, these arteries should be at or above 7 mm in diameter. The presence of tortuousity and calcification are factors that cause device deployment to be complicated and a potentially hazardous procedure.
In our experience, circumferential calcium, particularly in women, has been associated with adverse events (primarily, conversion to emergency open repair because of iliac disruption).
Pre-procedure Evaluation and Sizing of Graft
Lack of aneurysm exclusion is the ultimate consequence of mismatch between aneurysm size and graft size. Several modalities are used to estimate accurately:
- the diameter and length of the proximal and distal necks;
- the true length of the aneurysm and
- the diameter and length of the common iliac arteries (for bifurcated grafts).
Imaging modalities for accurate assessment include:
- Contrast enhanced Spiral CT scan of the abdomen and pelvis with 2.5-mm cuts. Options, including three-dimensional displays and maximal intensity projections, allow for more accurate assessment of the proximal aortic neck and assessment of the calcium content of the planned upper and lower attachment sites.
- A calibrated pigtail catheter during standard angiography enables,estimates about the length of the graft to be made. Three-dimensional rotational angiography eliminates errors that occur with tortuous aneurysms.(10) Our experience(11) suggests that the CT scan overestimates longitudinal lengths of the proximal aneurysm neck, the infra-renal aorta, as well as the iliac artery diameter. Currently, the contrast-enhanced spiral CT scan is the method of choice but rotational angiogrophy may well prove to be superior.
Defining Success in Endovascular Grafting
The theory behind endovascular treatment of AAA is exclusion of the aneurysm from the effects of systemic arterial pressure and thus preventing further enlargement and rupture. Ernst(2) defines exclusion as complete only when the graft is intact with no hole and the seal at both proximal and distal ends is complete.
After successful exclusion of the aneurysm by the graft, the aneurysm sac shrinks or stabilizes.(12),(13) Some studies(14) found that at 12 months only completely excluded aneurysms showed significant reduction in sizes (>6 mm in diameter), while those with an endoleak continued to dilate. Recent reports(15) suggest that the initial presence of endoleaks does not predict the lack of sac regression. The results of both studies would allow one to conclude that early endoleaks after endovascular grafting do not necessarily warrant aggressive treatment, whereas the presence of endoleaks at 6-month or 12-month follow up would be definite indications for further aggressive treatment (percutaneous coil embolization, covered stent or open repair).
Complications
- Conversion to open repair: With accumulating clinical experience, this rate has dropped to <5% (compared to an earlier 17%) with a peri-operative mortality rate of 11% that has declined from earlier values of 43 to 50%.(7),(16),(17),(18) The prognosis with open repair after failed endovascular grafting appears to be less favorable than elective open repair because of multiple reasons: First and foremost; following failure of endovascular grafting, open repair occurs in a patient who is more likely to be a poor surgical candidate. Second, it is a technically demanding procedure and, third, it comes in a context when the endovascular grafting failure has resulted in time consumption, blood loss and excessive use of contrast.
- Access site complications: The rigidity and diameter of the delivery device (18-F--24-F) make access site complications more likely. Dissection, disruption, perforation or rupture of the iliac arteries may occur.(19),(20),(21) These are more frequent with narrow, heavily calcified (especially, circumferentially calcified), tortuous iliac arteries.
- Endoleaks: An endoleak is defined as any persistent blood flow outside the vascular graft but inside the original intact aneurysm. Type I endoleaks occur at the proximal or distal attachment sites, while type II endoleaks occur in the setting of patent lumbar, intercostal or inferior mesenteric arteries. The accessory renal or internal iliac arteries may be sources of continued blood flow in to the aneurysmal sac. Type III endoleaks occur with tears in device material or incomplete seal of the contralateral limb of a bifurcated stent graft, resulting in continued flow in to the aneurysmal sac. Type IV endoleaks are self-limiting, since they represent arterial flow through suture lines or pores of the graft.(22),(23)
Continued flow into the aneurysm may result in further expansion of the aneurysm and rupture.(19),(24),(25),(26),(27) Endoleaks may be primary when they appear within 30 days of implantation of the device or secondary when they appear after initial complete sealing of the graft. Spiral contrast enhanced CT scan is the most accurate non-invasive test to detect endoleaks during follow up.
- Post implantation syndrome: onset of fever with temperatures between 38-39.70C, sweating and malaise characterize the syndrome.(28) Cultures tend to be negative. Most patients have impairment of the coagulation system (thrombocytopenia, reduced anti-thrombin III concentration and elevated fibrin degradation products). Reports of DIC (disseminated intravascular coagulation) with fatal outcomes after uneventful endovascular grafting have been documented.(29),(30)
- Renal artery obstruction: reports of the implantation of the endovascular grafting above the level of renal arteries without impairment of renal function are available.(31),(32) Gordon(33) reported deployment of a Gianturco stent across the level of renal arteries to attempt to secure fixation and optimization of the haemostatic seal at the cephalic end of the graft stent. It is generally true that deployment of uncovered stents across the renal ostia rarely lead to impairment of flow into the renal arteries. Obviously, malposition of a covered stent graft over the renal arteries is a major complication that almost always demands open repair with graft removal.
- Microembolization: potentially lethal complications secondary to microembolization may ensue, e.g., colonic or visceral ischemia with infarction and death.(34) Studies have demonstrated a higher risk of such complication in patients undergoing endovascular repair.(35) These complications are more likely with tortuous aneurysms which require excessive wire manipulation.
Conclusion
Endoluminal stent grafting is becoming an increasingly popular treatment modality for patients with abdominal aortic aneurysm. With accumulating clinical experience, complications following the procedure are becoming less frequent.
The role of endovascular treatment as a primary treatment modality in patients who are suitable for open repair has yet to be determined and little is known, to date, about the long-term implications of AAA repair with the available endovascular devices.