Course Authors
Eli A. Friedman, M.D.
Within the past three years, Dr. Friedman has received grant/research support from Alteon.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Classify and chart the course of proteinuria in the nephropathy complicating type 1 and type 2 diabetes
Discuss the timing and value of interventions that retard the progression of diabetic nephropathy, highlighting the value of drugs that impede the rennin-angiotensin system
Stage the course of diabetic nephropathy planning timing of therapy for chronic renal failure.
Over the past decade, diabetes has gained first place on the list of causes of kidney failure throughout the industrialized world. End-stage renal disease (ESRD) resulting from diabetic nephropathy is manifested by ~30% of individuals with type 1 diabetes and afflicts an undefined but probably equivalent subset of people with type 2 diabetes.
Currently, in the United States, Japan and industrialized Europe, diabetes mellitus is the leading cause of ESRD, surpassing glomerulonephritis and hypertension. As listed by the U.S. Renal Data System (USRDS) in 2001, of 344,094 U.S. patients receiving either dialytic therapy or a kidney transplant on December 31, 1999, 119,478 had diabetes (a prevalence rate of 33.3%).
Furthermore, during 1999, of 89,252 new (incident) cases of ESRD, 38,160 (42.7%) were listed as having diabetes. An additional 10% of incident ESRD patients had diabetes, though that was not the disease listed as having caused their renal failure, and a further 6% had the diagnosis of diabetes established during their first year of treatment for ESRD, raising the total proportion of diabetic ESRD patients to approximately 60% after one year. The actual reported lower prevalence for diabetic ESRD patients is explained for by their sharply higher death rate during ESRD therapy.(1)
Prior to 1980, diabetic patients were almost always excluded from ESRD treatment programs because initial reports of their course during dialytic therapy recounted excessive mortality and morbidity attributed to preexisting co-morbid conditions such as cardiovascular, cerebrovascular and peripheral vascular diseases. Pioneer reports of dialysis outcome in diabetics (primarily type 1 diabetic patients from the United States) described an unstable two-year survival ranging from 25-40%.(2),(3),(4)
In a composite registry report of the European Dialysis and Transplant Association in 1981, only 34% of diabetics survived three years of dialysis.(5) Although some improvement in survival of diabetic ESRD patients treated with hemodialysis or given a kidney transplant has been recorded over the last two decades, mortality of diabetics is still inferior to that of non-diabetics on dialysis.
Stages of Diabetic Pathophysiology
To understand the morbidity inflicted by diabetes, we need to review its stages and the interventions that may be applied. In both type 1 and type 2 diabetes, nephropathy has a well-described pathophysiologic progression. The sequence -- microalbuminuria with glomerular hyperfiltration, nephrotic syndrome, azotemia and end-stage renal disease -- is associated with the pathologic changes of mesangeal expansion, thickening of capillary basement membrane, nodular and diffuse intercapillary glomerular sclerosis, and afferent and efferent arterionephrosclerosis.
The previous thought that renal disease occurs more frequently in patients with type 1 than type 2 diabetes may be incorrect -- at least for Japanese adults. Yokoyama et al. found that in 1578 Japanese patients with onset of diabetes before age 30, 620 patients (39%) had type 1 and 958 (61%) had type 2 diabetes. Surprisingly, progression to clinical diabetic nephropathy was observed twice as frequently in those with type 2 when compared to those with type 1 diabetes. This finding underscored the point that type 2 diabetes is at least an equal threat to the kidneys, for these investigators noted a shorter interval from diagnosis of diabetes to development of nephropathy in the type 2 cohort than in those with type 1 diabetes.(6) Previously, in Minnesota, Humphrey et al. found renal failure equally probable over 30 years of direct observation in groups of 1,832 type 2 and 136 type 1 diabetic patients.(7)
ESRD Epidemiology
The incidence of ESRD with diabetes varies with age, race and type of diabetes. Blacks are 2.6-3.6 times more likely than whites to develop ESRD secondary to diabetes.(8) This relative risk of ESRD in black type 2 diabetics increases progressively with age, reaching a maximum that is 6.9 times higher than whites in those over the age of 65.(9) By contrast, type 1 diabetes mellitus imparts a relatively greater incidence of kidney failure in whites than in blacks, while type 2 diabetes mellitus has a relatively greater proportion affected with kidney failure in blacks than whites. Mexican Americans and Native Americans also have disproportionately higher incidences of diabetic nephropathy when compared with whites.(10),(11),(12)
Prospective, well-designed, controlled studies in diabetic patients have substantiated the beneficial renoprotective effects of several interventive regimens. Both development and progression of diabetic nephropathy are retarded by normalization of blood pressure,(13),(14),(15) blood glucose regulation(16),(17) and restriction of dietary protein.(18),(19),(20),(21),(22)
As is true for all medical regimens, what might be done and the actual delivery of effective care to the diabetic population at risk vary widely. For example, while the benefits of treating hypertension are ineluctably established, the majority of hypertensive Americans is presently treated inadequately. In this context, many diabetic individuals patients still progress to ESRD. Management of uremia in diabetes demands forethought and careful planning. To illustrate this point, three illustrative cases follow.
Patient 1: Microalbuminuria
A 19-year-old, type 1 diabetic woman, a college senior without complaint, volunteered for a community healthcare outreach screening program where microalbuminuria (according to postcard notification) was detected with a blood pressure of 121/73 mm Hg seated. Diabetes had been diagnosed at the age of 11 and a wearable insulin pump programmed by results of multiple daily finger-stick glucose measurements was prescribed. Over the past eight years, frequent visits to her pediatrician and for the past three years, to an internist, documented normal growth and development with a body mass index of 22.
Present findings include a normal physical examination including fundoscopy and a normal electrocardiogram. The hemogram and metabolic chemistry panel, including a serum albumin concentration of 4.1 g/dl, a blood urea nitrogen level of 9 mg/dl and a serum creatinine concentration of 0.8 mg/dl, were normal. A 24-hr urine collection discerned a creatinine clearance of 142 ml/min and a daily urinary albumin excretion of 67 mg. The hemoglobin A1c was 6.4 %.
Analysis and Action Needed
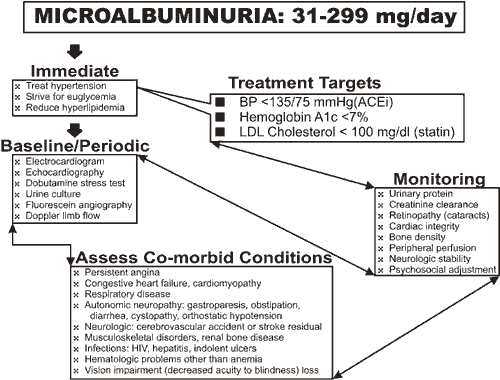
Microalbuminuria, a 24-hr urinary albumin excretion rate (UAE) of 20 to 200 micrograms/min (30-300 mg/24 h), is a laboratory finding predictive of subsequent nephropathy. The upper limit corresponds approximately to a total urinary protein concentration of 0.5 g/l, the hallmark of clinical nephropathy. Increased mortality is observed in both type 1 and type 2 diabetes with microalbuminuria. The usual course of diabetic nephropathy, however, entails months to years of a nephrotic syndrome (proteinuria >3.5 g/day, hypoalbuminuria, hyperlipidemia and anasarca) followed by azotemia, which signals the onset of CRF. ESRD is the typical termination of CRF.
Severe CRF induces myriad extra-renal diverse symptoms, physical signs and abnormal laboratory values that, in the aggregate, constitute the uremic syndrome. Appropriate therapy for the microalbuminuric and/or proteinuric individual with diabetes will retard progression of nephropathy, sometimes delaying ESRD for years.
Patient 1 affords perfect timing for productive intervention with a high likelihood of inducing maximal benefit. Microalbuminuria, defined as an amount of albumin secretion below that detected by dipstick, is an abnormal finding predictive of subsequent clinical nephropathy. Treatment with an angiotensin converting enzyme inhibitor (ACEi) such as benazepril, captopril, cilazapril, enalapril, enalaprilat, fosinopril, lisinopril, moexipril, perindopril, quinapril, ramipril and trandolapril will delay progression of microalbuminuria to clinical proteinuria (daily urinary albumin excretion >500 mg) and may retard pathologic changes of mesangial expansion and basement membrane thickening within the glomerulus.(23),(24),(25) Should an adverse reaction to the ACEi, such as nonproductive cough, force its discontinuance, equivalent benefit can be derived from an angiotensin receptor blocker (ARB) such as losartan, valsartan, irbesartan and candesartan.
Caution in the use of nephrotoxic drugs will avoid superimposed iatrogenic injury. Drugs that should not be administered to patients with azotemia include nitrofurantoin, spironolactone, amiloride, triamterene, metformin and phenformin. Abruptly deteriorating renal function may be the consequence of interstitial nephritis (not directly induced by diabetes) caused by captopril, cimetidine, methicillin sodium, allopurinol, phenylhydantoin, nonsteroidal antiinflammatory drugs and furosemide. Given a patient free of clinically expressed diabetic complications, a vigorous, proactive plan for protection against heart, eye and kidney disease should be initiated as shown in Figure 1.
The patient should be encouraged to become a member of the American Diabetes Association (ADA) where patient support groups can be identified. The prognosis for at least the next decade is excellent considering the normotension and attainment of the ADA target of a HbA1c <7% has been shown to be achievable in this patient. Should complications such as retinopathy, autonomic neuropathy or peripheral vascular disease be detected, consideration of a pancreas or islet cell transplant is appropriate long before renal function deteriorates to the level of clinical renal insufficiency.
Patient 2: Proteinuria
A 63-year-old mail carrier who has been treated for type 2 diabetes for 16 years complains of ankle swelling, inability to complete his route because of fatigue and dyspnea, as well as sexual impotence. On a regimen of oral hypoglycemic drugs (metformin and glycuride) and twice-daily finger-stick glucose testing, the HbA1c was 9.3%. Physical examination disclosed an alert, apprehensive, cooperative man with moderate obesity (BMI of 29), hypertension (BP = 163/95 mm Hg), background diabetic retinopathy, pitting edema up to the knees and moist basilar rales bilaterally. There were no other abnormal findings except for the absence of knee jerks bilaterally and a mild glove-stocking sensory neuropathy.
Laboratory tests indicated anemia (hematocrit of 32%), azotemia (BUN of 28 mg/dl, serum creatinine of 2.2 mg/dl), hyperlipidemia (total cholesterol of 331 mg/dl and LDL cholesterol of 145 mg/dl) and hypoalbuminemia (serum albumin of 2.9 g/dl). A 24-hour urine collection noted a creatinine clearance of 47 ml/min and a daily albumin secretion of 8.1 g.
Analysis and Action Needed
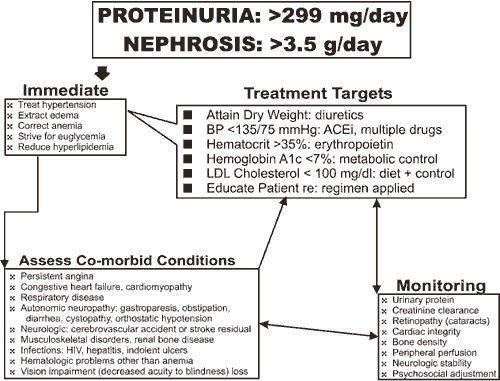
Patient 2 meets diagnostic criteria for the nephrotic syndrome (proteinuria >3.5 g/day, hypoalbuminemia, hyperlipidemia and edema) and also expresses diabetic retinopathy and neuropathy, fulfilling long-known components of the "triopathy of diabetes." The prognosis for further good health is poor, as the "accelerators" of diabetic nephropathy hypertension and hyperglycemia -- are both present and inadequately managed.
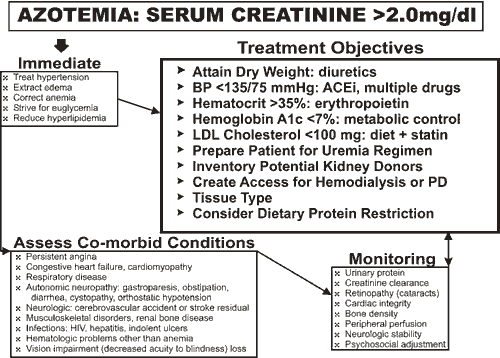
As was true for Patient 1, key interventions, especially strict regulation of blood pressure with an ACEi or ARB, will probably slow progression of nephropathy (Figures 2,3). It is too late for a preemptive pancreas transplant (that is not advised for type 2 diabetes except as an investigative procedure) and too early for a kidney transplant. The patient has entered a period of suspended animation, until the need for uremia" /> therapy is forced, that may be as short as six months and as long as five years.
Because of the risk of urinary infection, bladder catheterization in an azotemic diabetic patient should be restricted to the few instances when the information to be gained is unobtainable by other means. Facilitation of the measurement of daily urinary output is insufficient justification for an indwelling bladder catheter.
There is serious risk of worsening renal insufficiency when radiographic contrast medium is administered to diabetic patients with serum creatinine levels >2.5 mg/dl (>227.3 micromol/l). Under circumstances where radiographic contrast medium must be given, e.g., before a limb arterial bypass, prior hydration with half-normal saline and mannitol infusion (25 g in 2 L 0.45% saline solution) may protect against renal injury. Early trials in small numbers of diabetic patients suggest that pretreatment with N-acetylcysteine (600 mg orally twice daily), coupled with hydration with saline solution, will preempt contrast medium injury.
Though the patient has received suboptimal care until now, we cannot be assured that establishment of even an ideal regimen will be able to halt further deterioration over the short term. Immediate clinical improvement, consequent to removal of edema, will follow treatment with a combination of metalozone (10-30 mg twice daily) plus furosemide (40-160 mg twice daily).
To monitor diuresis and as a gauge for continuing dosage, a log listing blood glucose, morning weight and blood pressure should be started. With these data, adjustment of medication is simplified. Correction of the anemia of uremia is now a standard of care with target hematocrit levels near normal -- 35-40%.(26),(27),(28) A statin drug should be begun to reduce hyperlipidemia. Lastly, consultations with a cardiologist, ophthalmologist and podiatrist should be arranged promptly to modify a care regimen that will sustain Patient 2 through the coming storm of renal failure and its treatment.(29),(30)
Patient 3: ESRD
A legally blind 69-year-old woman from the Dominican Republic presented at a New York City Municipal Hospital emergency room with emesis, volume overload, anemia, uremia and a 7x5 cm foot ulcer that had been present for seven months. During the preceding year, weight loss of 45 lbs. (starting at 197 lbs. on a 5'4" frame), diminishing vision and chronic bilateral calf pain when attempting to walk had progressively restricted mobility to a bed and chair existence. The patient had minimal understanding of her medical condition, saw a clinic physician monthly and stopped taking "pills" for her diabetes two months earlier when she decided that they were of "no help." She had no blood or urine tests over the past three months.
On evaluation in the emergency room, the medical resident assessed Patient 3 as being profoundly and urgently ill. Her face and shoulders had precipitated uremic frost. She was tremulous, confused and dyspneic, breathing at a rate of 32. The blood pressure supine was 188/102 mm Hg, the pulse 114 and the temperature 97.5oF. Purpuric lesions were present throughout the skin. There were large bilateral cataracts and florid neovascularization with flame hemorrhages throughout the right retina. The left retina was not visualized. A harsh systolic and diastolic friction rub was heard over the entire precordium. Neither reflexes nor perception of pain were present in the lower extremities. Pedal pulses were not felt.
Laboratory findings included an hematocrit of 23%, a BUN of 296 mg/dl a serum creatinine of 27.5 mg/dl, a phosphorous of 10.3 mMol/L, serum albumin of 2.6 g/dl, a serum potassium of 7.2 mEq/L and a venous pH of 7.26. Plasma glucose was 348 mg/dl and the HbA1c was 12.2%. An electrocardiogram showed regular sinus rhythm, absent p waves, a wide QRS complex and tall tented T waves.
Analysis and Action Needed
Patient 3 represents desperation at the end of the line in diabetic nephropathy. At home in the Dominican Republic (or any of one hundred other nations lacking funds for modern ESRD therapy), Patient 3 would inexorably lapse into fatal uremic coma, probably within a month. By relocating to the United States and presenting herself to an emergency care facility, rescue therapy was assured.
With the initiation of an "acute" hemodialysis or peritoneal dialysis, Patient 3 enters the mainstream of patient flow -- from initial stabilization to selection of personalized treatment according to preference. As an undocumented alien, however, kidney transplantation is precluded, as the procedure and aftercare are not funded by either Medicare or Medicaid. Similarly, home hemodialysis is not feasible as neither machine purchase nor supplies will be supported by Medicaid. Wide variation between states determines how Medicaid will underwrite ESRD care for noncitizens. In New York, for example, repeated recertification for "Emergency Medicaid" at 30-day intervals is required to continue payments for ambulatory hemodialysis.
There are four main treatment choices (Figure 4) open to diabetic patients who lapse into ESRD:
- No further renal therapy (death)
- Peritoneal dialysis(31)
- Hemodialysis(32)
- Kidney (kidney plus pancreas) transplantation(33),(34)
Figure 4. Four main treatment choices for ESRD.
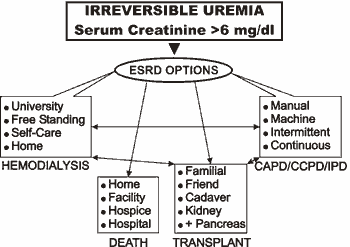
Matching an individual patient to optimized treatment requires consideration of personal preference as well as physical reality. As examples: solid organ transplants are unwise in a patient with unstable cardiovascular disease; peritoneal dialysis is preempted in the presence of an ileostomy; and home hemodialysis is impractical in the absence of a willing partner.
Recipients of a kidney transplant attain the best survival and most complete rehabilitation of any diabetic ESRD patients. It is possible that this superior outcome results from selection bias (cherry-picking) in choosing the most fit for a kidney transplant, leaving those with excessive comorbidity behind in the cohort treated by peritoneal dialysis or hemodialysis. An alternative explanation may lie in the improved metabolic milieu produced by a kidney transplant that extracts nitrogenous molecules such as advanced glycosylated end-products that are retained in "toxic" amounts by dialysis patients.
Throughout the past decade, first-year survival of diabetic ESRD patients, whether treated by dialysis or a kidney transplant, has improved each year. But survival after five years falls off precipitously in those treated by dialysis: fewer than 1 in 20 live ten years, whether treated by peritoneal or hemodialysis. Benefits of increased dose of dialysis and higher hematocrits afforded by erythropoietin and intravenous iron replenishment may become evident in subsequent registry reports.
As this Cyberounds® is written, a fresh approach to islet transplantation has gained attention because of the avoidance of steroids and the sequence of sixteen successes in a row in inducing freedom from insulin in type 1 diabetes.(35) But each recipient has required islets harvested from more than one donor (as many as four pancreases per recipient) immediately raising the problem of sufficient donor pancreases for broad application.
Furthermore, type 1 diabetes is responsible for no more than 5% of ESRD attributed to diabetes. Whether the type 2 diabetic individual will benefit from pancreas or islet transplantation is unknown. Not far down the road, however, growth of immortalized Beta cells and humanized porcine pancreata and islets may solve the supply dilemma, just as insertion of the insulin gene into yeast cells ended worry over an insufficient supply of insulin.