Course Authors
George V. Letsou, M.D., and Forrest Rubenstein, M.D.
Dr. Letsou is Associate Professor, Department of Cardiothoracic and Vascular Surgery, University of Texas Medical School, Houston. Dr. Rubenstein is a cardiothoracic and vascular surgery, Toledo, OH.
Drs. Letsou and Rubenstein report no commercial conflict of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss normal and abnormal mitral valve function
Enumerate the differences between the primary types of mitral valve repair
Describe the differences between types of annuloplasty rings.
Mitral valve disease and mitral regurgitation, in particular, constitute some of the most difficult management challenges in cardiovascular medicine. The evolution of mitral valve repair for patients with mitral regurgitation has provided a physiologically attractive alternative to valve replacement with a prosthetic mitral valve. This is particularly true for patients who have remained in sinus rhythm, since repaired valves do not require long term anticoagulation. Mitral valve repair also preserves the physiologic anatomy of the ventricle and allows the heart to eject more efficiently than rigid circular valves which restrict the pumping efficiency of the left ventricle. Drs. Letsou and Rubenstein have assembled a very useful and interesting description of mitral valve repair and what we can expect from this novel new cardiac surgical technique. Please welcome them to Cyberounds®!
-- Richard W. Smalling, M.D., Ph.D. Cardiovascular Moderator
Surgery for valvular heart disease has undergone dramatic advances over the past five decades. Early attempts at mitral valve surgery were aimed at correcting valve stenosis and employed various "closed" techniques of mitral commissurotomy (mechanical dilation being the most common). These techniques had inconsistent outcomes and were largely abandoned because of the significant problems with induced mitral regurgitation secondary to overaggressive dilation and restenosis.
Gibbon's introduction of cardiopulmonary bypass in 1953 allowed for direct visualization of the mitral valve and stimulated interest in mitral valve repair and replacement. The first attempt at correction of mitral insufficiency by annuloplasty was in 1957 by Lillehei at the University of Minnesota. Dr. Nina Braunwald performed the first successful implantation of a prosthetic mitral valve in 1959 at the National Institutes of Health. The Starr-Edwards mechanical "ball and cage" prosthetic valve became commercially available in the early 1960s. Mitral valve replacement soon became the procedure of choice for mitral stenosis and mitral insufficiency because of the operation's ease and the reproducibility of results.
Many other mechanical valves were introduced over the next 20 years with improved hemodynamic profiles (i.e., lower gradients across the valve), with lower profiles (i.e., more compact and easier to fit within the left ventricle) and with improved long-term performance. However, mechanical valves still had significant disadvantages including thromboembolism, trans-valvular gradients, hemolysis, infection, peri-valvular leaks and mechanical failure. They also required anti-coagulation and its attendant complications. The introduction of a porcine bioprosthesis in 1969 ameliorated many of these complications and eliminated the need for anti-coagulation. However, the durability of bioprostheses was less than anticipated and resulted in the need for serial valve replacement surgery, especially in younger adults and children.
Repair Instead of Replacement
The less than satisfactory results with bioprosthetic and mechanical valves stimulated interest in techniques for repair rather than replacement. Perhaps the most important advance was an improved understanding of normal mitral valve function. Carpentier developed a classification detailing anatomic changes in the valve leaflets, in the mitral annulus and in the sub-valvular apparatus underlying mitral insufficiency.(1),(2) He described three types of mitral insufficiency: Type I has normal leaflet motion with annular dilation or leaflet perforation; Type II has prolapsed leaflets; and Type III has restricted leaflet motion.
Mitral insufficiency in Type I is caused by a central jet of insufficiency secondary to either the annular dilation or perforated leaflets. Insufficiency in Type II is caused by an elongated chordae, a ruptured chordae or a ruptured papillary muscle, all leading to one leaflet prolapsing more than the other, poor leaflet coaptation and production of an asymmetric jet of insufficiency. In Type III, insufficiency results from commissural fusion and leaflet thickening or associated fused chordae preventing leaflet coaptation.
Based on his classification, Carpentier proposed several repairs including patching of leaflet perforations, shortening of chordae to restore normal valve coaptation, transposition and/or replacement of chordae to improve leaflet coaptation, and the insertion of an "annuloplasty ring" to restore normal annular diameter and prevent further annular dilation.
The improved understanding of the pathology underlying annular dilation was critically important in developing durable mitral valve repairs. It was recognized that annular dilation predisposing to mitral insufficiency occurs only along the posterior (non-septal) portion of the annulus. The septal portion of the annulus, anchoring the anterior leaflet, does not enlarge. Thus, annuloplasty was directed at shortening the annulus underlying the posterior leaflet. Annuloplasty rings are now designed with shortening of the annulus underlying the posterior leaflet as the prime objective. All surgical repairs of the mitral valve emphasize the importance of appropriate annular sizing and reinforcement.
Mitral Valve Anatomy
The human mitral valve has two leaflets, anterior (or aortic) and posterior (or mural). The anterior leaflet composes roughly two thirds of the valve area and is shaped like a truncated triangle. The posterior leaflet is more elongated and rectangular. The posterior leaflet usually has three scallops separated by small clefts. The anterior leaflet is attached to the septum and fibrous annulus of the heart and thus is relatively non-distensible. Although the anterior leaflet accounts for 2/3 of the mitral valve area, its attachment to the mitral annulus comprises only approximately 1/3 of the mitral annulus circumference. The anterior mitral valve leaflet spans the distance between the two fibrous trigones and is in direct fibrous continuity with the non-coronary aortic valve leaflet. The posterior mitral valve leaflet is attached to the posterior 2/3 of the mitral annulus, which runs along the free wall of the left ventricle and is primarily muscular with little fibrous tissue (accounting for its tendency to distention and elongation). The anterior and posterior leaflets are fused for 3 to 8 mm medially and laterally at the trigones and usually form very distinct commissures.
Quadrangular Resection With An Annuloplasty Ring
The most common mitral valve repair performed is quadrangular resection of the posterior leaflet with placement of an annuloplasty ring. This repair is performed using standard cardiopulmonary bypass techniques including cannulation of both superior and inferior vena cavae with complete diversion of blood flow to the cardiopulmonary bypass circuit providing a blood-free operating field. Moderate systemic hypothermia and complete aortic occlusion with standard cardioplegic techniques are used.
The left atrium is opened from the superior pulmonary vein to the inferior vena cava parallel to the interatrial groove. The heart is then retracted up and to the patient's left. Placing a laparotomy pad on the anterior surface of the heart facilitates exposure of the anterior mitral valve leaflet. Placing a laparotomy pad behind the heart, displacing the heart's posterior surface anteriorly, facilitates exposure of the posterior mitral valve leaflet.
Examination of the mitral valve and assessment of pathology is the critical portion of any repair. Both anterior and posterior leaflets are manipulated using a blunt hook in order to delineate pertinent abnormalities. When a ruptured chordae is identified with concomitant lengthening of its attached posterior mitral valve scallop, quadrangular resection must be considered. Extensive calcification of the annulus or leaflet makes successful repair less likely.

Figure 1-3. Figure Headline.
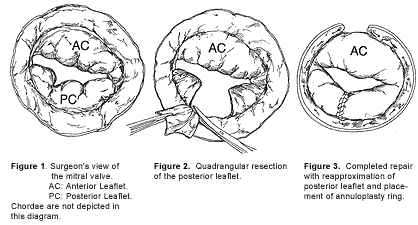

Quadrangular resection is performed after identifying the prolapsing scallop. A rectangular portion of the posterior leaflet, including this scallop and extending from the leaflet's edge to the annulus, is excised. Any ruptured chordae and portions of the neighboring scallop up to, but not including, intact neighboring chordae are also excised. This composes the "quadrangular resection." The remaining portions of the posterior leaflet are then sewn to one another using fine, usually 5-0, Gortex suture which is softer than polypropylene and does not cause leaflet perforation. The annulus is reapproximated with heavier 2-0 sutures. The annular portion is the repair's Achilles heel. This area is reinforced with 2-0 sutures beginning at the annulus and extending on to the atrium itself taking care to avoid the underlying circumflex artery.
An annuloplasty ring of appropriate diameter is chosen after examination of the anterior leaflet and annulus. The anterior leaflet is retracted and pulled towards the posterior leaflet, exposing the anterior portion of the annulus. The various companies manufacturing annuloplasty rings have developed annuloplasty ring "sizers." The sizers measure the anterior leaflet annulus length from commissure to commissure (which, as discussed above, does not lengthen as the entire annulus dilates) and allow choice of a ring with appropriate diameter. The many types of commercially available rings are detailed below. The ring is sewn to the annulus, taking particular care to reinforce the weakest part of the repair, the posterior annulus in the region of the quadrangular resection.
There are many different techniques to test the repair but all involve filling the ventricle with fluid and examining the repair for incompetence and leaks visually. Once a satisfactory repair has been obtained, the left atrium and left ventricle are filled with fluid and the left atrium is closed. Standard techniques are used for weaning from cardiopulmonary bypass. The repair is then again examined closely using transesophageal echocardiography under various loading conditions after the patient is weaned from cardiopulmonary bypass. An experienced echocardiographer is an essential member of any team performing mitral valve repairs. If any significant mitral regurgitation is detected, it is repaired at this point.
Chordal Shortening and Transposition Techniques
Mitral insufficiency may also be secondary to lengthening of the chordae. Repair of this abnormality is also performed using standard cardiopulmonary bypass techniques, cannulation of both superior and inferior vena cava, full cardiopulmonary bypass, systemic hypothermia, aortic occlusion and cardiac arrest. The left atrium is opened and the valve examined using the techniques described previously. In addition to the techniques for exposure of the anterior and posterior leaflets described above, a laparotomy pad may also be placed behind the apex of the left ventricle. This facilitates exposure of the anterior and posterior papillary muscles and their attached chordae. Exposure and analysis of these chordae is the most important portion of the procedure.
Assessment of pathology when chordae are lengthened or redundant can be more difficult than when chordae are ruptured. The left ventricle and left atrium take on different conformations when the heart is empty. The preoperative echocardiogram is an extremely helpful guide to the surgeon's correct analysis of pathology. Chordae and papillary muscles are examined using a blunt hook to expose them while the left ventricle is empty. The ventricle is then filled with cold saline to mimic conditions when the heart is full. Portions of the mitral valve which leak are more closely examined. Scallops which prolapse and leak are those to which elongated chordae are attached.
The objective of repair is elimination of valve prolapse by appropriate "shortening" of elongated chordae. "Shortening" is accomplished at the base of the chordae where it arises from papillary muscle. A cleft is created in the papillary muscle body taking care not to injure the chordae. The chordae is then shortened by placing a portion of it within the cleft and closing papillary muscle over the chordae using permanent suture. The technique shortens chordae by placing the excess chordae within the papillary muscle.
The annulus is reinforced with an annuloplasty ring chosen by measuring the anterior annulus, as described above. Filling the left ventricle with fluid tests the repair. The patient is weaned from cardiopulmonary bypass and the valve is further examined using transesophageal echocardiography. Any incompetence must be corrected at this point.
Another technique of chordal repair is replacement of damaged or elongated chordae. These techniques have the disadvantage of introducing foreign material. However, when chordal shortening is not possible because the chordae has been irretrievably damaged, the use of artificial chordae is important. Artificial chordae are cut to an appropriate length (as determined by filling the left ventricle) and then sewn from the edge of the valve leaflet to the appropriate papillary muscle. Such chordae give excellent long-term results.
"Chordal transposition" is another technique used primarily in anterior mitral leaflet repair. When chordae attached to the anterior leaflet of the mitral valve are ruptured, translocation of a posterior chordae to the anterior leaflet is a preferred method for repair. (Anterior leaflet quadrangular resections have been unsuccessful.) Such chordal transposition repairs are performed when examination of the incompetent mitral valve reveals leakage from the anterior leaflet secondary to chordal rupture.
The damaged portion of the anterior leaflet is excised along with its underlying malfunctioning chordae. A chordae from the posterior leaflet opposite the damaged anterior chordae is chosen as a replacement. This portion of the posterior leaflet is detached as a free chordae with attached valve leaflet. The defect in the posterior leaflet is repaired using the quadrangular resection, as described above. The free chordae and its attached portion of posterior leaflet are sewn into the anterior leaflet defect using fine 5-0 Gortex suture. An appropriate annuloplasty ring reinforces the repair. Once again, the repair is tested by filling the left ventricle with cold saline and, subsequently, using transesophageal echocardiography once weaning from cardiopulmonary bypass is complete.
Annuloplasty Alone
Cardiomyopathies and heart failure often result in cardiac dilatation, an enlarged ventricle, a dilated annulus, and, therefore, mitral insufficiency. Improvements in the management of heart failure have resulted in increasing numbers of such patients coming to surgical attention. These patients often have moderate to severe mitral insufficiency in association with cardiac failure. Usually, the mitral insufficiency manifests as a central regurgitant jet secondary to annular dilation caused by inability of the anterior and posterior leaflets to coapt centrally.
There is increasing experience in repairing such pathology using an annuloplasty ring alone. For such repairs, meticulous cardiac protection and peri-operative management of heart failure are mandatory. Mortality may be high unless stringent indications for surgery are followed. Medical and surgical teams experienced in the care of heart failure patients must be involved peri-operatively.
Standard cardiopulmonary bypass is used, sometimes in association with intra-aortic balloon pump counterpulsation pre-operatively. Cannulation of both inferior and superior vena cavae are performed with moderate systemic hypothermia, aortic occlusion and standard cardioplegic arrest techniques. One challenge is the advanced bi-atrial and bi-ventricular enlargement often seen in cardiac failure. Cardiac manipulation to expose anterior and posterior mitral valve leaflets can be challenging.
A surgical approach through the right atrium and atrial septum should be considered in cases of dramatic cardiac enlargement. Once the left atrium is opened, the ventricle is filled with cold saline and the presence of a central insufficient jet is confirmed. The anterior leaflet annulus is measured and an annuloplasty ring of appropriate diameter is placed (although some authors report excellent results with routine placement of a 28 mm ring).
Overcorrection of insufficiency (and resultant mitral stenosis) is more prevalent after these repairs and must be excluded with intra-operative transesophageal echocardiography. Patients with cardiac failure tolerate mild mitral insufficiency relatively well and a perfect result is not mandatory in this patient population. The long-term results of mitral valve repair in cardiac failure are less extensively documented than for other etiologies of mitral insufficiency. Nevertheless, for appropriate patients, such repairs lead to marked improvements in lifestyle.
Repair of Mitral Valve Perforations
Endocarditis often results in perforations of the anterior and posterior mitral valve leaflets. Some perforations are amenable to repair with pericardial patches. In these cases, autologous pericardium is harvested prior to initiation of cardiopulmonary bypass. Once cardiopulmonary bypass is initiated and the left atrium has been opened, the valve is inspected for vegetations and/or perforations. In many instances, especially in untreated cases, extensive vegetations have destroyed much of the anterior or posterior leaflet and such cases are not appropriate for repair. In other patients, especially those who have been partially treated, the vegetations are smaller, not extensive, and the only manifestations of endocarditis are smaller perforations of the leaflet. In these cases, harvested pericardium is cut to an appropriate size and used to patch defects after resection of all affected leaflet.
Pericardium can also be used to repair partial disruptions of the valve leaflet from the annulus caused by endocarditis. The repair can be reinforced with an annuloplasty ring fashioned from pericardium and/or an overlapping suture technique of 3-0 polypropylene (both reinforce the annulus without foreign material). These repairs are particularly beneficial as they involve the introduction of little or no foreign material in an infected field.
Commissurotomy for Mitral Stenosis
Mitral stenosis is also amenable to repair using long-established techniques for commissurotomy. Repairs of this type are performed with cannulation of both vena cavae, aortic occlusion, moderate systemic hypothermic and cardioplegic arrest. When the left atrium is opened, calcified and distorted anterior and posterior mitral leaflets with the thickening associated with rheumatic heart disease are found. The commissures are fused. Division of the commissures to the level of the annulus results in significant relief of mitral stenosis in some patients.
Most surgeons, however, prefer mitral valve replacement in cases of pure mitral stenosis because results are superior. For most patients with pure mitral stenosis, commissurotomy has been abandoned in favor of prosthetic valve replacement. Nevertheless, division of the commissures can be essential when repairing mixed lesions of mitral insufficiency and stenosis.
Selection of Annuloplasty Rings
As experience with mitral valve repair increased, and as the anatomy and physiology of the mitral valve and annulus were better understood, numerous new annuloplasty rings were developed. There are currently more than 25 different annuloplasty devices including rigid rings, flexible rings, rings that are rigid anteriorly and flexible posteriorly, rings that only provide posterior stabilization, various suture annuloplasty and plication techniques instead of rings, rings that can be tailored and cut to various lengths, rings that are adjustable after termination of bypass, "homemade" rings fashioned from Dacron grafts, PTFE vascular grafts or pericardium, and absorbable rings for pediatric valve repair.
The purpose of all annuloplasty rings or devices is to reduce the size of the mitral orifice, allow for better leaflet coaptation and stabilize repairs done on the valve leaflets or support structures. There are occasional mitral valves with small annuli or isolated lesions, such as ruptured chordae and perforated leaflets, without annular dilation that do not require any annuloplasty ring at all.
Initially, annuloplasty rings were rigid and widely employed with great success. The Carpentier-Edwards rigid ring allows for a measured plication of the entire annulus and is sized according to the length of the anterior leaflet annulus (the intertrigonal distance). It essentially immobilizes the posterior leaflet and, theoretically, fixes the mitral valve at the optimal size for the ventricle in systole. It also forces the annulus to remain fixed in a horizontal plane. If the ring is oversized, it can cause systolic anterior motion (SAM) of the anterior leaflet, left ventricular outflow tract obstruction and require conversion to valve replacement.
With a better understanding of these potentially unfavorable mitral valve dynamics and an improved understanding of the importance of the annulus and subvalvular apparatus on ventricular function, the limitations of a rigid annuloplasty ring become more concerning. The mitral annulus is a dynamic structure that moves in three dimensions, has a non-planar shape and a sphincter action. Flexible annuloplasty rings (Duran, Cosgrove-Edwards, Sculptor, BiFlex and others) are designed to allow for normal movement and function of the annulus. These flexible rings allow a measured reduction of the annulus without forcing it into a planar shape or interfering with the reduction in annular cross-sectional area during systole. Vertical mobility and augmentation of ventricular contraction by the subvalvular apparatus is also maintained.
Comparison of flexible and rigid rings using transesophageal echocardiography has documented that flexible rings interfere less with normal mitral valve motion, improve peak velocity across the ring, improve left ventricular fractional shortening, improve left ventricular end-diastolic diameter and improve left ventricular end-diastolic volume.(6),(7) Studies of the Cosgrove-Edwards flexible ring using echocardiography from 6-24 months after repair confirm appropriate sphincter function of the mitral valve and a decrease of 19% in the mitral valve orifice during the cardiac cycle (from 10.3 cm2 in diastole to 8.6 cm2 in systole). Alternatively, rigid rings do not effectively change their orifice area.(8) Nevertheless, despite these hemodynamic improvements, reproducible improvements in survival for patients receiving flexible rings over those receiving rigid rings have not been documented.
Since only the posterior portion of the mitral annulus dilates significantly, several "partial" flexible annuloplasty rings have been designed that allow for measured plication and stabilization of the posterior annulus but are theoretically easier and quicker to insert since no sutures have to be placed in the anterior annulus. In addition to decreasing bypass time, these rings avoid some of the potential problems associated with placing sutures along the anterior annulus including injuries to aortic valve leaflets, to the anterior mitral leaflet and to the AV node. An example of this type of partial ring is the SJM-Seguin annuloplasty ring.
There are adjustable annuloplasty rings, which were designed to allow for adjustment of the annular length during valve testing (Puig-Mason) and even an externally adjustable device, which can be adjusted after termination of bypass using transesophageal echocardiography guidance. There is, as yet, no long-term data to support claims that these rings offer any objective improvement over other flexible rings.(9),(10)
Various types of "homemade" rings fashioned from material easily available in the operating room have also been advocated. Such rings are economical and durable. Examples include those fashioned from woven Dacron tube grafts and from PTFE vascular grafts.(11) Published reports show similar results to other flexible rings although long-term follow-up is limited.
Recently, various types of suture plication of the posterior annulus have been reported. These techniques involve a measured plication of the posterior portion of the annulus using over-and-over sutures. Mortality rates are low. Functional results are excellent and the technique shows considerable promise. Follow-up is currently inadequate (12 months) to fully evaluate the durability of these repairs.
Autologous pericardial posterior annuloplasty has been utilized in several centers in Italy since the early 1990s. There are two centers reporting 5-year follow-up with good mitral valve function and stability by echocardiography and clinical parameters. The operative mortality rate is low (2.7%) and 95% of patients are in NYHA Class I after repair. The 5-year event free survival is 91%. Prospective long-term follow up is underway. This repair avoids the use of prosthetic material and offers distinct advantages in repair of infected valves.(12),(13)
Summary
Mitral valve repair must be considered in all patients with mitral insufficiency. Perioperative and long-term morbidity and mortality are reduced compared with prosthetic valve replacement except in patients with rheumatic heart disease. Quadrangular resection of the posterior leaflet with annuloplasty ring insertion is the most commonly performed repair and offers excellent long-term function without the need for anticoagulation. Chordal shortening, transposition and replacement with annuloplasty ring insertion also offer excellent long-term results. Annuloplasty ring placement alone is being performed successfully in heart failure patients. Flexible rings appear to give the same support as rigid rings and have similar long-term durability but do not compromise dynamic annular and ventricular function; hemodynamic parameters are greatly improved but there is no clear survival advantage of flexible over rigid rings.