Course Authors
Flora M. Vaccarino, M.D.
Dr. Vaccarino is Associate Professor at the Child Study Center, Yale University School of Medicine.
Dr. Vaccarino reports no commercial conflict of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Discuss how the key characteristics of neural stem cells make them potentially important for promoting regeneration
Outline the differences between embryonic and adult neural stem cells
Describe some of the extrinsic factors that regulate the proliferation and survival of neural stem cells.
In the last decade, neuroepithelial cells have been isolated in vitro(1),(2),(3) that can undergo self-renewal and are capable of giving rise to the principal cells types of the brain, including neurons, astrocytes and oligodendrocytes. Neurons and oigodendrocytes are the principal signaling components, while astrocytes provide nutritional and metabolic support.
These neuroepithelial cells can be isolated from the mouse, rat, monkey and human central and peripheral nervous system both during embryogenesis and adulthood.(4),(5),(6),(7) These progenitor cells have been called "neural stem cells." Stem/progenitor cells isolated from the hippocampus or the telencephalic subventricular zone (SVZ), a neuroepithelial cell layer lining the cerebral ventricular space throughout the life span, are able to populate other brain regions upon transplantation, suggesting a broader developmental potential.(8),(9)
These studies have suggested that the normal adult central nervous system contains neural stem cells that are capable of integrating into different sites and perhaps give rise to a large variety of mature cell types. Hence, the prospective has been opened of using these cells to reconstitute CNS regions that are not properly developed or lost as a result of acute trauma or degenerative disorders. Several problems will have to be resolved before stem cells can be subjected to clinical applications. The first is to delineate the true potential of these cells to fully differentiate not only in vitro, but especially in vivo.
Neurogenesis in the Postnatal Brain
It has been more than 40 years since it was discovered(10),(11) that neurogenesis occurs in vivo in the adult mammalian dentate gyrus in the hippocampus. This structure is located within the hippocampus, a brain region that is critical for the encoding and recollection of recent information. De novo production of hippocampal granule cells has now been confirmed in a variety of species, including primates and humans.(12),(13) More recently, neuronal progenitors have been identified in the adult SVZ; these cells give rise to new neurons that migrate to the olfactory bulb.(14),(15),(16),(17)
Apart from these two regions, the dentate gyrus and olfactory bulb, evidence for generation of new neurons by endogenous "stem cells" in physiologically intact mammalian CNS is scanty and highly controversial.(48),(49),(50) There could be multiple reasons for the lack of an obvious neuronal replacement in regions such as the spinal cord and the cerebral cortex. For example, it is possible that neuronal replacement is not easily detectable under the "deprived" conditions of caged laboratory animals. It is also likely that neural stem cells become progressively restricted in their potential with increasing age of the organism. Therefore, adult neural stem cells would differ from their embryonic cousins in that they are unable to generate all neuronal fates.
The reason for this difference between adult and embryonic stem cells is unclear, and it could be intrinsic to the cells themselves, meaning that stem cells are programmed to give rise to certain cell types at defined steps during their development, and, therefore, actually "age" as they become more restricted in their fate. Alternatively, the cells could be multipotential, but could be prevented from differentiating in certain cell types in the adult brain because of the lack of instructive or permissive cues in the environment. The answer to these questions is not known, since the fate of embryonic and adult stem cells in an in vivo environment has not been adequately examined.
Thus, the central issue is whether neural stem cells, isolated from the adult CNS, are truly multipotential and whether we can identify potential obstacles to their differentiation and integration in the mature brain tissue.
Embryonic Stem Cells
Embryonic stem cells (ES cells) that develop from the inner cell mass of the early postimplantation embryo are totipotent cells, i.e., these cells give rise to all tissues of the developing organism. Neural tissue arises within a restricted area of the ectoderm by a suppression of the epidermal fate in ES cells. Transforming Growth Factor ß (TGF- ß) signaling actively promotes epidermal differentiation. In addition, Fibroblast Growth Factors (Fgf) are required from the very onset to promote differentiation within the neural lineage.(18),(19),(20)
Much before definitive nerve cells are born, the antagonism of TGF- ß signaling and the local release of Fgf receptor agonists allow the emergence of generic progenitors of the neural lineage, or primitive neural stem cells. While the molecular mechanisms underlying this Fgf/ TGF- ß antagonism are not completely clear, these two signaling molecules act on different intracellular transduction systems and activate different sets of target genes. For example, Fgf would cooperate with other unidentified factors to induce the expression of nuclear transcription factors that "switch on" generic programs of neural gene expression.
With further development, neural progenitor cells throughout the CNS become geographically subdivided into compartments by regulatory genes that confer regional identities and regulate the size of these compartments.(21),(22) Many of these genes are transcription factors of the homeodomain class, a large family of evolutionarily conserved transcription factors isolated about 40 years ago in Drosophila. Homeodomain transcription factors confer unique cell properties which make cells stick together and forbid intermix of adjacent groups of cells expressing expressing different homeodomain genes.
Furthermore, homeodomain genes expressed in adjacent compartments can be mutually exclusive because they repress each other. Thus, the process of ES cell differentiation is characterized by differential expression of transcription factors by daughter cells and their spatial segregation. The result is the creation of regional neural cell "pools" each characterized by a unique code of transcription factor activity. Transcription factors direct genetic programs that specify cell fates, growth and neuronal connections within progenitor cells and their neuronal progeny that progressively migrate to their target sites.
Consistent with this progression, primitive neural stem cells developing directly from ES cells in the early postimplantation embryo have a wider potential, as they can contribute to many tissues in all germ layers upon transplantation. In contrast, neural stem cells isolated at later embryonic stages (e.g., embryonic day 8.5 from the mouse anterior CNS) have lost this potential.(20) Neural stem cells isolated from the postnatal brain are able to generate only restricted neuronal types, granule cells and interneurons of the olfactory bulb. Thus, neural stem cells may undergo a progressive fate restriction and differentiation.
These considerations strongly suggest that the majority of neural progenitor cells are committed to a certain regional fate, but it could still be hypothesized that a small minority of stem cells maintains a higher degree of fate potential. This might explain why in vitro expanded adult stem cells, which are presumably enriched for these putative multipotential progenitors, have been reported to generate highly divergent cell lineages, such as muscle or blood.(23),(24) However, stem cells are expanded using two growth factors that are necessary for their proliferation and survival: Epidermal Growth Factor (EGF) and basic Fgf (Fgf2).(27) Therefore, one could just as easily hypothesize that Fgf2 and Egf, the two growth factors that are used to foster their in vitro expansion, are altering their fate potential. Hence, the lack of neurogenesis in most regions of the adult CNS may be due to a non-permissive environment, e.g., the lack of growth factors which maintain stem or progenitor cells' multipotential. This possibility should be tested by transplanting embryonic multipotential neural stem cells into various adult CNS regions and determining their fate. Certain non-CNS environments, such as the bone marrow, may maintain characteristics similar to the embryonic CNS.
Adult Stem Cells
As mentioned above, new neurons arise continually from stem and progenitor cells in two specialized forebrain germinal areas: the hippocampal subgranular layer and the SVZ.(25) Because stem cells capable of giving rise to both neurons and glial cells in vitro have been isolated from virtually every adult brain region, including white matter, the capability of generating nerve cells may be latent throughout the CNS.(26)
Stem cells can be isolated from many embryonic and adult CNS regions using EGF and Fgf2, two peptide growth factors binding to receptor tyrosine kinases that are expressed on the membrane of neural progenitors and other cell types. Intraventricular infusions of Fgf2 increase cell proliferation in the adult SVZ.(28),(29) However, while Fgf2 treatment during embryogenesis promotes the generation of pyramidal neurons in the cerebral cortex that contain the excitatory transmitter glutamate, in the adult it promotes the generation of small inhibitory neurons that migrate to the olfactory bulb.(30) This suggests that the neuronal progenitors in the adult SVZ are no longer specified for the production of pyramidal neurons, that is, they are no longer competent to respond to Fgf2 by up-regulating the same set of target genes.
Although "stem cells" from the adult SVZ are able to produce "generic" neurons and glia in vitro, their potential to produce specific types of CNS neurons such as glutamate-containing cortical neurons, dopaminergic neurons or hippocampal pyramidal neurons has been apparently lost. These neuronal types have been obtained only after isolation of stem/progenitor cells from their prospective embryonic CNS regions.(31),(32) Both excitatory and inhibitory neurons are important for neural information processing in the CNS, but the hippocampal excitatory neurons tend to be lost in hypoxia/ischemia, dopamine neurons in Parkinson's and cortical pyramidal neurons in Alzheimer's disease.
However, a portion of SVZ cells die and only a subset normally mature and differentiate into neurons in vivo; therefore, the lack of generation of pyramidal cells in the adult could be due to selective factors. That is, the cerebral cortex may be no longer permissive for the differentiation and maturation of progenitor cells unless special circumstances arise locally. An intriguing question that has recently emerged is whether the lost competence of stem cells can be reversed by instructive factors present in the stem cell environment.
If the cause of progenitor death and lost competence to generate a wide variety of neuronal types turns out to be the presence of inhibitory signals from mature neurons and other CNS cells, we can hope to reverse this process in the adult brain. Some evidence for this hypothesis has been given by recent results describing the generation of new pyramidal cortical neurons after the induction of neuronal apoptosis (cell death) in the mouse cerebral cortex.(33)
Local Regulation of Neural Stem Cell Proliferation and Survival
The division, differentiation and survival of neural stem cells are subjected to extrinsic regulation by the local microenvironment and the systemic hormonal milieu. For example, stress hormones and excitatory amino acids released during neuronal activity suppress neural stem cell proliferation in the hippocampal granular zone. The negative effect of stress and adrenal hormones on hippocampal neurogenesis is mediated by a stimulation of NMDA receptors presumably on mature granule cells.(34),(35) Possibly, this can explain the hippocampal atrophy found in patients suffering from depression and post-traumatic stress disorder.(36),(37),(38),(39)
Stem and progenitor cell proliferation is stimulated by conditions in which there is neuronal apoptosis, such as acute hypoxia/ischemia.(40) Furthermore, it appears that cognitive factors affect progenitor cell survival in the hippocampus. Many more new neurons will survive from these progenitors in an enriched environment or in conditions of active learning.(41),(42)
Because excitatory NMDA receptors and glucocorticoid receptors are not on progenitor cells, but on mature neurons, these results suggest that an intermediary cell must mediate the influence of mature neurons on progenitor cells. Astrocytes are glial cells that are present throughout the CNS and exert important metabolic and neurotransmitter functions, such as the metabolization of glucose, the conversion of glutamate to glutamine, the channelling of calcium and potassium ions, and they may intervene in synaptic plasticity. Because astrocytes are profoundly affected by neuronal activity, the inhibitory effects of neuronal activity on neurogenesis may be mediated by the network of these glial cells. This raises the question of whether chronic stress may alter cell turnover in the hippocampus and may predispose to hippocampal cell loss through an action on glial cells.
It has been recently proposed that stem cells belong to the astroglial lineage.(43),(44) It has been suggested that mature astrocytes are able to transform into radial glia, a more primitive form of astroglia, if exposed to soluble factors present in the embryonic brain.(45) Finally, an Fgf2-responsive astroglial precursor cell isolated in vitro has been proposed to act as a stem cell in the adult brain.(26) Interestingly, a subset of astrocytes expresses Fgfr-2 in their nucleus. Furthermore, the enhanced cell proliferation in the neurogenic zones induced by chronic hypoxia is accompanied by a transformation of astrocytes into radial glia.(46)
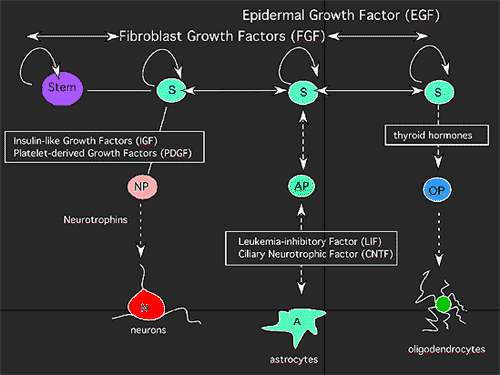
Radial glia are progenitor cells for cortical pyramidal neurons normally present in the ventricular germinal layer during cortical neurogenesis; these cells divide, generate cortical neurons and support their migration to the developing cortical plate. Recent live imaging experiments in embryonic cortical slices have shown that radial glial cells "bud off" neurons which then climb onto the radial fibers and migrate to the developing cortex.(47) It is possible to envision a process whereby local conditions of cell loss or other perturbations allow mature glial cells to "revert" to their embryonic counterpart, the radial glia, which under appropriate conditions would regenerate new pyramidal neurons (Figure 1).
Developmental time line outlining the progression between a primitive neural stem cell, its development into a stem cell primarily generating neuronal progenitors (NP) in particular CNS regions, and finally a stem cell primarily generating astrocyte progenitors (AP) and oligodendrocyte progenitors (OP). Some of the factors that control the differentiation and maturation of these cells are shown. Although there is a temporal distinction between these different types of stem cells, their distinct molecular characteristics are hypothetical. Note that astrocytic cells can "revert" into cells capable of generating neurons.
Future experiments should test whether the hypothesized reversion of mature astrocytic cells to radial glia is accompanied by a respecification of their fate; that is, whether these cells are potentially able to regenerate lost pyramidal cells or other cell types. This could be tested by transplanting stem cells/radial glia isolated from the adult SVZ into the embryonic cortical ventricular zone.
Conclusions
During CNS development, the restriction of multipotential stem and progenitor cells into specialized pools of neuronal progenitors is regulated by region-specific transcription factors. Because cellular differentiation is tightly coupled to the region of the neuroepithelium from where the cell develops, genes that regulate CNS pattern formation may be key for stem and progenitor cells to develop their unique neuronal phenotypes. It remains to be seen whether neural stem cells can escape the set of genetic programs that are operative in the intact CNS and whether they can be induced to re-program themselves into a variety of regional cell types.
Multiple factors influence the proliferative and differentiative potential of neural stem cells, including neuronal activity. An intriguing idea is that these factors affect the reversible interconversion of astrocytes into radial glia, a form of immature progenitor. The question naturally remains, as to the potential of radial glial cell to generate a wide variety of neuronal cells. This question will be tackled by future experiments examining the fate of radial glial cells in both the embryonic and the adult mammalian brain. Answering these questions will depend on future advances in our understanding of the genetic programs and environmental cues that guide the birth, maturation and migration of different neuronal types.
Acknowledgments
Research in the author laboratory on some of the topics discussed in this article is supported by NIH grants R01 NS37709-01 and PO1 MH49351, NSF grant IBN-0083104 and by the NARSAD foundation.