Course Authors
Frank A. Laws, M.D., and Richard W. Smalling, M.D., Ph.D.
Dr. Laws is a Fellow in the Department of Internal Medicine, Division of Cardiology, University of Texas-Houston Medical School.
Dr. Laws reports no commercial conflict of interest. Dr. Smalling has received grant/research support from Centocor/Boston Scientific within the last three years.
This activity is made possible by an unrestricted educational grant from the Novartis Foundation for Gerontology.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Identify why blockade of the renin-angiotensin aldosterone system (RAAS) confers a beneficial effect in patients with congestive heart failure
Identify pathways involved in the production of angiotensin-II and receptors that mediate the effects of angiotensin-II
List the objectives of ongoing clinical trials assessing the role of ARBs in the treatment of patients with congestive heart failure.
Congestive heart failure (CHF) is a clinical syndrome characterized by a limitation of exercise tolerance (due to dyspnea and/or fatigue), which can be attributed to or physiologically related to an identifiable abnormality of cardiac function.(1) Heart failure is a common problem, affecting approximately four million people of all ages in the U.S., and is more frequent with advancing age.(2) The Framingham study demonstrated an average of 3.7/1000 men and 2.5/1000 women diagnosed yearly with cardiac failure.(3) Currently, over 400,000 new cases of heart failure occur each year in the U.S. and the treatment of heart failure accounts for over 17 billion dollars in medical expenses per year.(4)
The prognosis of subjects diagnosed with severe heart failure is poor and efforts are under way to develop pharmacologic interventions which will improve this prognosis.(5) The high yearly mortality gives rise to an all-cause mortality rate of approximately 70-80% over a five year time interval.(6) The advance of therapies, targeted to reduce acute ischemic cardiovascular mortality, may alter the epidemiology of congestive heart failure, as more subjects survive acute ischemic cardiovascular events to later experience progressive heart failure.
The Renin-Angiotensin Aldosterone System
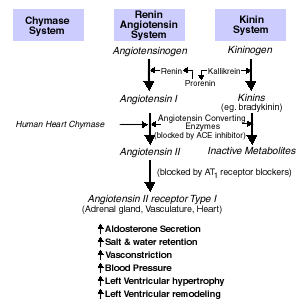
The activation of the renin-angiotensin aldosterone system (RAAS), which leads to the production of angiotensin-II (Ang II), has been implicated in the pathogenesis and progression of heart failure.(7) Over the last two decades, the use of angiotensin converting enzyme inhibitors (ACE-I) has led to improvements in cardiac function and a decrease in hospitalization and mortality in patients with New York Heart Association (NYHA) functional class I to IV heart failure.(8),(9) The beneficial effects of ACE-I have been linked to their ability to block the production of angiotensin-II (Ang-II), a potent vasoconstrictor which promotes cardiac remodeling.(10) ACE-I has also been shown to block the breakdown of bradykinin (Figure 1), which in animals has led to the prevention of adverse cardiac remodeling.(11)
Angiotensin II Receptors
Ang-II exerts its effects by stimulating specific receptors on the plasma membrane of different target organs. To date, four types of Ang-II receptors have been characterized.(12) In humans, the type-1 (Ang-II) receptor [AT-1] and the type-2 (Ang-II ) receptor [AT-2] predominate.(13) Although both receptor types appear in different organs in varying proportions, it seems that most, if not all, known cardiovascular effects of Ang-II are mediated via the AT-1 receptor.(13) Despite maximal pharmacologic blockade with ACE-I, many patients on chronic therapy have readily detected levels of Ang-II in the plasma and tissues. It has been demonstrated that other tissue-specific serine proteinase inhibitors, such as chymase, are capable of converting angiotensin-I to angiotensin-II, independent of ACE Activity (Figure1).(14) Several investigators have shown, in vitro, that most of the formation of Ang-II in normal and failing hearts is due to chymase activity and not ACE activity.(15),(16)

Figure 1.

Angiotensin II Receptor Blockers (ARBs)
These observations, along with the fact that several adverse effects may be associated with ACE-I therapy (cough and angioedema), stimulated the quest to develop other approaches to pharmacologically inhibit the RAAS. The development of Ang-II type-1 receptor blockers (ARBS) provides another approach to counteract the deleterious effects of Ang-II on the cardiovascular system. At present, there are five ARBs approved by the FDA for use in the treatment of hypertension (candesartan, eprosartan, irbesartan, losartan and valsartan).
When compared to ACE-I and other groups of antihypertensive agents, the ARBs have been shown to have equal efficacy in the reduction of left ventricular hypertrophy and diastolic blood pressure, and also to be well tolerated.(17),(18)
The ability to block the effects of Ang-II generated by the RAAS without the suppression of bradykinin has made the idea of administration of ARBs in heart failure an attractive one. Theoretically, the combination of an ACE-I and an ARB could confer incremental benefits not seen with the usage of either group of agents by themselves.(19) To date, the FDA has not approved any ARB for treatment of heart failure. However, in small clinical trials, losartan was demonstrated to have beneficial hemodynamic effects during both short-term and long-term therapy. In other studies, the administration of ARBs did not improve exercise tolerance or confer the same survival benefit, when compared with placebo and oral ACE-I respectively.(20)
In an effort to clarify the role of ARBs in the clinical management of heart failure patients, several large clinical trials have been initiated. The results of the ELITE and RESOLVED trials have since been published with the ELITE-II, ValHeft and CHARM trials in progress.
Clinical Trials
ELITE (The Evaluation of Lorsartan in the Elderly Study) Lancet 1997;349:747-52
This was a randomized trial of lorsartan versus captopril in patients over 65 to determine whether specific angiotensin II receptor blockade offers safety and efficacy advantages in the treatment of heart failure over ACE inhibition.
722 patients with NYHA class II-IV heart failure and ejection fraction of 40% or less were randomized to lorsartan (n = 352), 50mg per day or captopril (n = 370), 50mg p.o. t.i.d. for 48 weeks. The primary end point was the tolerability, with the secondary end points of death and/or hospital admission for heart failure.
Data from this study indicated that lorsartan had better overall tolerability than the ACE inhibitor, captopril, in the elderly patients not previously exposed to an ACE-I. Additionally, patients on lorsartan had an improvement in survival when compared to captopril (46% reduction in mortality rate: from 8.7% to 4.8%), confidence interval [CI], 5 to 69; p = 0.035). The survival benefit appeared to be primarily attributable to a reduction in sudden cardiac death (lorsartan,1.4 % vs. captopril, 3.8%: risk reduction = 64%; 95% CI, 3 to 86).(21)
In an effort to clarify the effect of lorsartan on sudden cardiac death, the Lorsartan Heart Failure Survival Study ELITE-II is now ongoing.(22) It is a multicenter, double blind, randomized clinical trial to formally test the hypothesis that lorsartan, when compared with captopril, will reduce all-cause mortality (primary endpoint) and sudden cardiac death and/or resuscitated cardiac arrest (secondary end-point).
RESOLVD (The Randomized Evaluation of Strategies for Left Ventricular Dysfunction) Circulation. 1999;100:1056-1064
This was a multicenter, double blind, randomized parallel placebo controlled pilot study designed to compare the effects of candesartan, enalapril and their combination on exercise performance, ventricular function, quality of life (QOL), neurohormones and tolerability. A secondary goal was to identify the optimal dose of candesartan.
768 patients with an ischemic or nonischemic dilated cardiomyopathy and mild-to-moderate heart failure were randomized to candesartan (up to 16 mg/day), enalapril (up to 20 mg/day) or a combination of both drugs, which were added to conventional therapy for 43 weeks. After 19 weeks, eligible patients were further randomized to metoprolol or placebo.
When the three groups were compared, there was no significant difference in exercise tolerance. There was a trend towards increased ejection fraction in the candesartan/enalapril group. With respect to CHF and mortality, there was a trend to increased events in the candesartan and the combination group [14.6 % for candesartan, 15.1% for candesartan plus enalapril and 3.7% for enalapril alone (three-way group comparison p = 0.048)]. Despite not being powered to detect the effect of candesartan on mortality, the trend towards an increased number of events is of concern.(23)
Other Clinical Trials
Other clinical trials currently in progress include the Valsartan Heart Failure trial (Val-Heft) and the Candesartan in Heart Failure-Assessment of Reduction in Mortality and Morbidity trial (CHARM).
The primary objectives of Val-Heft are to investigate the effects of valsartan, compared with placebo, on morbidity and mortality, signs and symptoms and QOL in patients with chronic heart failure treated with ACE-I with or without the background of beta-blocker therapy.(24)
The CHARM trial is designed to investigate the clinical usefulness of the long-acting ARB candesartan, cilexetil, in a broad spectrum of patients with symptomatic heart failure. The study will evaluate the effect of candesartan on all cause mortality and CHF hospitalization.(25)
Conclusion
Despite trials showing good tolerability of ARBs in CHF patients, the results of completed trials (ELITE and CHARM) are inconclusive. The authors of the recently published Consensus Recommendations for the Management of Chronic Heart Failure made the following recommendations with respect to the use of ARBs in heart failure:
- There is no persuasive evidence that angiotensin II receptor antagonists are equivalent or superior to ACE inhibitors in the treatment of heart failure. Therefore, angiotensin II receptor antagonists should not be used for the treatment of heart failure in patients who have no prior use of ACE inhibitors and should not be substituted for ACE inhibitors in patients who are tolerating ACE inhibitors without difficulty.
- Because of the lack of conclusive evidence supporting the efficacy of these drugs in heart failure, it is reasonable to prescribe angiotensin II receptor antagonists instead of ACE inhibitors only in patients who are intolerant of ACE inhibitors due to angioedema or intractable cough.(26)
With the above in mind, it appears that the defined role of ARBs in the treatment of patients with heart failure will have to await the results of ongoing clinical trials.