Course Authors
Gary M. Gray, M.D.
Dr. Gray reports no commercial conflict of interest.
Estimated course time: 1 hour(s).

Albert Einstein College of Medicine – Montefiore Medical Center designates this enduring material activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
In support of improving patient care, this activity has been planned and implemented by Albert Einstein College of Medicine-Montefiore Medical Center and InterMDnet. Albert Einstein College of Medicine – Montefiore Medical Center is jointly accredited by the Accreditation Council for Continuing Medical Education (ACCME), the Accreditation Council for Pharmacy Education (ACPE), and the American Nurses Credentialing Center (ANCC), to provide continuing education for the healthcare team.
Upon completion of this Cyberounds®, you should be able to:
Analyze the history of a patient with GERD and establish a systematic approach to the diagnosis and therapy
Discuss the pathophysiology of intestinal metaplasia (Barrett's epithelium) in the lower esophagus
Recognize the factors that increase the risk of developing Barrett's epithelium
Determine when a patient with Barrett's should be referred to a gastroenterologist or surgeon.
Esophagitis
Gastro-esophageal reflux disease (GERD) is a very common entity caused by exposure of gastric acid to the lower esophagus because of incompetence of the lower esophageal sphincter that allows gastric acid regurgitation. Typical symptoms are a burning sensation in the anterior chest, substernal pressure and a sour taste related to the regurgitation of gastric contents.
The squamous epithelium responds to the bathing acid by summoning lymphocytes and neutrophils to the sub-epithelial layers. The resulting mucosal damage and loss of the surface squamous cells then promotes rank ulceration, fibrosis and stricture formation. Although symptoms are often most prominent after the patient retires, daytime regurgitation is predominant in many patients. Pain over the sternum as the food bolus passes (odynophagia) and a sensation of lodging of ingested solid foods (dysphagia) may develop as the esophagitis becomes chronic. The esophagitis produces persistent and disturbing regular symptoms over many years in about three-fourths of patients with the disease. At least half of these patients experience heartburn on a daily basis and one-third of them require daily drug therapy.(1)
Although esophagitis is often refractory to the conventional therapy of elevation of the head of the bed and reduction of acid secretion with antacids or H2-receptor antagonists, the advent of the proton pump inhibitors (PPIs) (omeprazole or Prilosec®; lansoprazole or Prevacid®) has allowed much better medical therapy of the disease. Whereas esophagitis is known to be resistant to healing by antacids or H2-receptor antagonists, one or two capsules per day of omeprazole or lansoprazole provide relief from heartburn and sour regurgitation and promote healing of erosive lesions within four weeks in the vast majority of patients. Indeed, at least two times as many patients obtain relief and healing with the PPI therapy when compared to those treated with the H2-receptor blockers.(2),(3)
The PPIs actually are less expensive in severe esophageal acid-reflux disease than the H2-receptor antagonists because the H2 receptor blockers often need to be administered in twice the dose used for duodenal ulcers.(4) The cost of a single daily dose of omeprazole (20 mg) or lansoprazole (30 mg) is $3 to $4. Lansoprazole 30 mg/day is equivalent to omeprazole 20 mg in the therapy of severe esophagitis(5) and a lower dose of either drug (lansoprazole, 15 mg or omeprazole 10 mg) may be sufficient in some patients to maintain remission from stubborn esophagitis.
Barrett's esophagus
Despite the improvement in therapy of esophagitis, the alterations in the lower esophagus, caused by chronic regurgitation of gastric contents, are now recognized to predispose to the development of an adenocarcinoma of the lower esophagus. This risk of malignancy is related to the tendency of the squamous mucosa of the esophagus to be replaced by migrating columnar cells from the upper stomach (cardia). The gastric-type mucosa, placed under considerable stress in its new environment, responds by developing numerous goblet cells, acquiring the characteristics of intestinal tissue, the so called intestinal metaplasia. It is commonly called Barrett's epithelium and has the propensity, over several years, to evolve into a true dysplasia and, eventually, to an adenocarcinoma.
The risk of developing Barrett's esophagus is related to the history of regular symptoms of gastro-esophageal reflux, the advancing age (> age 50) of the patient and the presence of inflammation of the squamous epithelium related to acid reflux.(6),(7),(8) Barrett's esophagus is identified in 10% of patients who undergo upper endoscopy for symptoms of GERD. The risk of malignancy due to intestinal metaplasia of the columnar lined lower esophagus is 30 to 125 times that in the esophagus without the Barrett's changes.(9) Indeed, this carcinoma, at the esophago-gastric junction, has displayed a dramatic increase in incidence in recent years to become the commonest malignancy of the upper gastrointestinal tract, especially among Caucasian males. The search for and surveillance of Barrett's esophagus has been intense over the past several years and the physician should be aware of the evolution of the diagnostic and therapeutic choices that are now available.
What Constitutes Barrett's Esophagus?
In the broadest definition, Barrett's can be considered to be the replacement of any amount of the squamous epithelium of the lower esophagus with an altered columnar epithelium displaying intestinal metaplasia that has an enhanced potential for proliferation and eventual progression to dysplasia and cancer.(10) But this is too simple a definition because both the topography and the degree of the metaplasia are very important when considering its potential for progression to dysplasia and carcinoma. Intestinal metaplasia of the columnar epithelium of the stomach, whether occurring in the antrum, fundus, or cardia, is also common and does not hold the high potential for malignant change as that of intestinal metaplasia of the esophagus.(11) Typically, endoscopy reveals an irregular squamo-columnar junction with finger-like projections of salmon-pink colored columnar mucosa that has migrated up into the gray-colored squamous lining of the esophagus (Figure 1). When these projections continue for 3 cm or more, it is said to constitute Long Segment Barrett's Esophagus (LSBE), and there is a close correlation of the severity and length of the patients symptoms with the pathologic findings of intestinal metaplasia. In contrast, involvement of shorter lengths of esophagus (< 3 cm; Short Segment Barrett's Esophagus or SSBE) is often not associated with reflux symptoms and has a lower incidence of intestinal metaplasia than LSBE that is similar to that found for a discrete, normal appearing gastro-esophageal mucosal junction.(12)
Figure 1. Comparison of Normal E-G Junction (Left; Opening to Stomach in Center) with Well Defined Junction of Gray-colored Squamous Mucosa of the Esophagus with the Salmon-pink Mucosa of the Stomach.
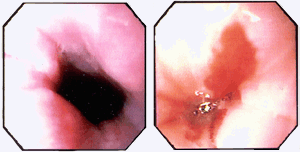
In contrast, the mucosal junction from a patient with Barrett's epithelium (right, entrance to stomach covered by highlights from water bubbles) reveals an elongated tongue of salmon-pink mucosa consisting of intestinal metaplasia extending 3 cm into the distal esophagus.
Considering the differing behavior of intestinal metaplasia as a function of its topographic location, intestinal metaplasia is more likely to be found when replacement of the lower esophageal squamous lining extends for 3 cm or more with extensive bands or islands of the salmon-colored columnar epithelium. The mere existence of metaplasia at a normal appearing squamous-mucosal junction or metaplasia associated with short projections of salmon-pink mucosa in the lower esophagus does not have the same implications for the development of dysplasia and carcinoma.
Evolving Tools in the Diagnosis of Esophageal Intestinal Metaplasia
Because the projections of salmon-pink extensions of columnar tissue seen at endoscopy correlate only moderately well with histologic intestinal metaplasia (the endoscopic diagnosis is verified by the histologic findings in 55% of identified LSBE and in only 25 % for SSBE),(13) additional tools have been sought to enhance the accuracy of the endoscopic diagnosis of Barrett's esophagus. Moreover, some of these may enhance establishment of the diagnosis of intestinal metaplasia.
Methylene Blue, for example, preferentially binds to metaplastic tissue, so application of this dye via the endoscope facilitates the selection of the altered tissue for biopsy.(14) Alcian Blue staining of the recovered fixed tissue identifies the altered columnar goblet cells typical of metaplasia. The intestinal antigens, such as Villin(15) CK 7 and CK 20,(16) not present in normal esophageal epithelium, are present in more than 75 % of specimens with esophageal intestinal metaplasia. Although the standard for diagnosis of intestinal metaplasia is the identification of the typical microscopic changes by the pathologist, the finding of metaplasia in the nearby cardia weakens the significance of the metaplastic tissue at the esophageal-gastric junction. Metaplasia in the gastric cardia is more commonly associated with metaplasia elsewhere in the stomach and with infestation of Helicobacter pylori organisms(17),(*),(*) factors that do not correlate with esophageal intestinal metaplasia, dysplasia and carcinoma. For this reason, it may be advisable for the endoscopist to obtain a sample from the gastric as well as from the salmon-pink projections suggesting metaplasia in the lower esophagus.
Implications of Barrett's Esophagus
At first analysis, Barrett's can be considered to be a severe form of esophagitis and hence the mainstay of therapy at the outset is the potent reduction of acid secretion afforded by the proton pump inhibitors, omeprazole and lansoprazole. In the setting of severe esophagitis, double-dose PPI therapy is often necessary to eliminate symptoms of GERD and a prokinetic agent, such as cisapride (Propulsid®), may also be added to reduce the residence time of acid in the lower esophagus. Unfortunately, this is often not successful in reversing the Barrett's. Even though laparascopic fundal plication, whereby a roll of the upper stomach is affixed about the distal esophagus, has been found to be very useful for preventing GERD, it is not established that this procedure eliminates the intestinal metaplasia in such patients.
Because there is an appreciable risk that the Barrett's intestinal metaplasia will evolve into a true dysplasia and, then, to carcinoma, the existence of typical Barrett's in the lower esophagus is an indication for monitoring the patient for the development of dysplasia. This can be done most efficiently and with minimum risk by a surveillance endoscopy and biopsies of the lower esophagus every 24 to 36 months. When dysplasia does develop, resection of the lower esophagus and a gastric pull-up or jejunal interposition is indicated because as many as half of patients who develop dysplasia will have a focus of frank carcinoma identified in the resected specimen.(*) Hence, patients in whom dysplasia is identified should be monitored with a repeat endoscopy and multiple biopsies of the involved lower esophagus every three months (for severe dysplasia) to every six months (for mild dysplasia) in an effort to identify carcinoma as early as possible.
Non-surgical Eradication of Barrett's Esophagus
Besides the monitoring of Barrett's esophagus with surveillance endoscopies and treatment to reduce acid production and reflux, other approaches have been studied in a limited number of patients. Laser ablation of the unwanted columnar intestinal metaplasia can be accomplished in 60-80 % and dysplasia and superficial carcinomas may also be eliminated.(*) However, in a significant minority of patients in whom the metaplasia is successfully ablated, intestinal metaplasia is maintained beneath the newly formed squamous layer(*) and the extent and frequency of recurrence of the metaplasia are not yet known.
Guidelines in the Approach to the Patient with GERD
This is provided below in tabular form. In general, the key for many patients is the marked advantage of PPI therapy over H2-receptor antagonists or alkali antacids. However, a trial of the H2-blockers is worthwhile before proceeding to PPI therapy. Supportive measures of relatively small low fat evening meals and raising the head of the bed may reduce the G-E reflux but often are not sufficient to control symptoms. The physician should be aware of the limitations of medical therapy. Acid suppression relieves symptoms, and heals the esophagitis, but this does not appear to reverse the Barrett's or to prevent its progression to dysplasia and carcinoma.
Table 1. Therapy of the Stages of GERD.
| Category | Description | Recommended Therapy |
| Mild heartburn only | Occasional bouts for periods of a week or longer |
|
| Moderately severe heartburn |
|
PPI single dosage x 6 weeks |
| Recurrence and persistence of moderately severe GERD symptoms | Rapid recurrence of symptoms after course of PPI Rx; occasional odynophagia/dysphagia |
|
| Severe, persistent GERD symptoms | Daily symptoms, often with dynophagia; dysphagia |
|
| Recalcitrant GERD symptoms |
|
|
| Development of Barrett's epithelium | [same as in severe or recalcitrant GERD] |
|
| Development of dysplasia, carcinoma in situ | [same as in severe or recalcitrant GERD] |
|
Musings About the Future for GERD and Barrett's
GERD is a very common entity but the frequency of Barrett's in mild GERD or in patients with few or no symptoms is unknown. Gastroenterologists have become involved, increasingly, with the care of GERD patients because of the risk of metaplastic changes that may progress to dysplasia and carcinoma. At present, the usual advice is to have all patients with Barrett's epithelium undergo surveillance upper endoscopy every 12 months, especially in the older patient (> 50 years). This represents a formidable task and expense of monitoring patients.
Non-invasive, efficient methods of diagnosis are needed to define the actual risk of development of Barrett's in the patient with mild disease. Other than developing new, efficient and safe techniques for making an early diagnosis and monitoring the metaplasia, perhaps the most challenging aspects of the Barrett's metaplasia --> dysplasia --> carcinoma sequence are prevention and early recognition that would be facilitated by development of non-invasive methods that would lead to local, non-mutilating therapy before the disease becomes beyond control.
General Review
Goyal RK et al. Columnar Lined (Barrett's) Esophagus. Clinician 17 (6):1-30, 1999.